Redox Titration_Tadas_09
advertisement

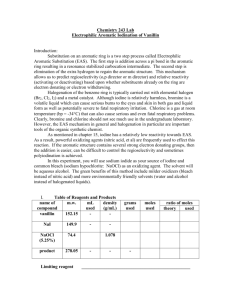
Analysis of Laundry Bleach: An Oxidation-Reduction Titration Tadas Rimkus AP Chemistry Period 2 Background Information Reduction-oxidation reactions describe all chemical reactions in which atoms have their oxidation number (oxidation state) changed. Oxidation describes the loss of electrons Reduction describes the gain of electrons Background Information Background Information Titration is a common laboratory method that is used to determine the unknown concentration of a known reactant. A reagent, called the titrant, of a known concentration (a standard solution) and volume is used to react with a solution of the titrand, whose concentration is not known. Objectives SWBAT: Calculate the percentage of bleach in common bleach products. Learn to complete titration labs Learn about reduction-oxidation reactions Reactions You will add an excess of potassium iodide (KI) to an acidified sample of laundry bleach. The NaOCl in the bleach oxidizes the I- ion to I2. The amount of I2 produces is directly related to the original amount of NaOCl (or OCl- ions) present in the bleach: 2I- + OCl- + 2H+ I2 + Cl- + H2O Reactions You will titrate the I2 produced in Reaction 1 with a standardized solution (known molarity) of sodium thiosulfate (Na2S2O3) as the titrant. Thiosulfate ion reduces the I2 ion to I- ion by the following redox reaction: I2 + 2S2O3 2I- + S4O62- The indicator is starch solution, which forms an intensely blue complex with I2. This color vanishes at the end point when the last trace of I2 is reduced. Materials Commercial laundry bleach KI, 2M Acetic Acid, 6M Na2S2O3 5H2O Starch indicator, 1% 500-mL Volumetric flask Stirring rod Funnel 50-mL buret 250-mL beaker Distilled water Procedures 1. 2. Rinse and fill your cleaned buret using the sodium thiosulfate solution (Na2S2O3). Record the initial volume to the nearest 0.01 mL. Clean and rinse a 250-mL Erlenmeyer flask with distilled water. Dry the outside and weigh to the nearest milligram. Procedures 1. 2. Add 2.0-2.5 mL of bleach to the weighed flask (remove flask from scale before adding bleach) Reweigh the flask to the nearest milligram. www.clorox.com Procedures 5. Immediately after second weighing, add 100 mL of distilled water (pour it down the sides of the flask to wash down any bleach drops) www.npwd.org www.merriam-webster.com school.discoveryeduc ation.com 5. 6. Measure out 10 mL of dilute (6 M) acetic acid (HC2H3O2) and 8 mL of 2 M KI Add the acetic acid to the flask, swirl, and then add the KI solution and swirl. upload.wikimedia.org vitaminsbeautycare.com www.merriam-webster.com Procedures 8. 9. 10. 11. Titrate promptly by slowly adding titrant from the buret, swirling the flask constantly. When the solution has changed to a gold-orange and then to a faint yellow color, add 20 (1 mL) drops of starch indicator to turn the solution blue. Rinse the inside surface of the flask with distilled water. Place a piece of white paper under the flask and continue to titrate until the blue color just barely disappears. Procedures 1. 2. 3. 4. 5. Record the final buret reading. In your notebook, calculate the ration R = (Volume of titrant delivered)/(Mass of bleach) in units of mL/g. Refill the buret, record the buret reading, and titrate an additional bleach sample following the steps above. Calculate R for the new titration When finished, drain the volumetric flask and buret and rinse. Results Mass of Na2S2O3 2.40 g Bleach brand name Clorox Bleach Solution 1 Solution 2 Mass of Bleach (g) 2.39 g 2.42 g Volume of titrant delivered 34.36 mL 34.51 mL Molarity of thiosulfate solution .9710 M .9714 M Moles of Sodium Thiosulfate used .09710 mol 0.09714 mol Moles of Iodine reduced .007 mol .0082 mol Moles of NaOCl in sample .0312 mol .0327 mol Mass of NaOCl in sample 2.322 g 2.434 g Percent NaOCl by mass 12.37% 12.51% Conclusion By calculating the two percentages of the bleach samples, we got 12.37% and 12.51% bleach by mass for Solution 1 and 2 respectively. This completed our first objective. And by completing this experiment, we learned how to do titrations and learned about oxidationreduction reactions.