Equilibrium Controlled Reactions:
advertisement

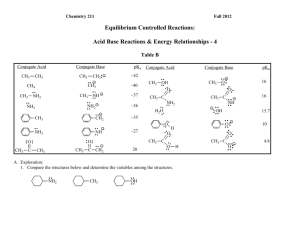
Chemistry 211 Fall 2010 Equilibrium Controlled Reactions: Acid Base Reactions & Energy Relationships - 4 Table B Conjugate Acid CH4 : : : : NH2 NH ~35 O : :O : ~27 CH3 20 C O : : : CH3 OH : OH 10 :O : H 16 15.7 O: A. Exploration: 1. Compare the structures below and determine the variables from one structure to another. NH2 NH CH3 C - O: : : CH3 CH3 - H : : C C CH2 NH2 16 :O : CH3 C H2O : O: :O : CH3 - CH3 C O: : : CH3 :O : pKa : : CH2 CH3 OH : : ~36 CH3 : : : : : NH2 - NH - : : : NH3 ~40 ~37 CH3 NH2 Conjugate Base ~42 : : : CH3 CH2: CH3 : CH3 Conjugate Acid : : CH3 pKa : : CH3 Conjugate Base 4.8 Acid-Base Rxns & Energy Relationships-4 2 3 Acid-Base Rxns & Energy Relationships-4 2. Use the data in Table B to list the compounds in 1. in the order of decreasing acidity and provide a warrant supporting your claim citing specific data in Table B. (As usual, more data supporting a warrant makes the claim stronger.) Most Acidic Middle Least acidic Warrant: 3. Which of the following ions has the lowest energy? Which has the highest energy? Provide a warrant supporting your claims and citing specific data in Table B. (As usual, more data supporting a warrant makes the claim stronger.) O Lowest Energy NH C CH2 : O: C : : - : : C O O Highest Energy Warrant: B. Suggest theoretical backing to explain the effects observed above in terms of electron nuclear attraction and/or electron-electron repulsion. Acid-Base Rxns & Energy Relationships-4 4 5 Acid-Base Rxns & Energy Relationships-4 C. Using the theoretical backing developed in Part B, select the most acidic proton(s) in the following compound. Then provide a warrant describing the process and logic that led to your claim. H H H H C C H H C H N C O C C H H H H H H Reflector’s Report Discussion: Identify the most important concepts you learned from this activity: What questions remain? Strategy Analyst’s Report Discussion: A. Exploration: What role did question 1 play in the exploration of Table B in question 2? What Principle or Assumption that wasn’t necessary for question 2 was required to answer question 3? B. Theoretical Backing: Acid-Base Rxns & Energy Relationships-4 6 What fundamental aspect of the variable identified in question A. 1. was the key to providing the backing needed in B.? How was it used? Out of Class Applications for Acid Base Reactions &Energy Relationships - 4 A. Reading Assignment: in CGWW: pp. 181-191. B. Activities: 1. CGWW has a subtitle “An acid’s pKa depends on the stability of its conjugate base.” (p. 187) We described acidity in terms of the energy of the conjugate base. In this context, what is the relationship between a base’s energy and its stability? Provide a warrant to support your claim. 2. Problem 4, in CGWW, CH 8: p. 207, first 2 molecules. : : : 3. For each of the following compounds, use the theoretical backing developed in this activity to select the most acidic proton(s): H H H H H H H N C C O H H H H C N C O C H H C C H H C H H H H H Then provide a warrant describing the process and logic that you used to reach your conclusions. : : : 4. Which of the following ions should have the lower energy. - - : : CH2 : : CH2 P H S: CH3 CH2 CH2 Provide a warrant describing how you used the theoretical backing developed in this activity to devise your claim. CH3 7 For each of the following acid-base reactions, use the theoretical backing in this activity to predict whether the equilibrium constant should be > or < 1. Provide a warrant describing the process and logic that you used to reach your conclusions. a. : O : : b. CH2 : O: - H 6. : + : + NH2 : : + : NH H + CH3 O - : : : : 5. Acid-Base Rxns & Energy Relationships-4 O H H For the following pair of equilibrium controlled reactions, predict which one should produce more product at equilibrium. Explain your choice on the basis of the theoretical backing developed in this activity. -S O + + O S -S NH - - HN S Acid-Base Rxns & Energy Relationships-4 8 C. Nomenclature of Ethers 1. CGWW: Ch. 2 p. 32 2. Tutorials: a. http://chemistry.boisestate.edu/people/richardbanks/organic/nomenclature/organicnomenclature1.htm Developed by Richard C. Banks, Professor of Chemistry, Boise State University Section Ethers Provides questions with answers b. http://www.molecularmodels.ca/nomenclature/index-2.htm Developed by Professor Dave Woodcock, Sections: Okanagan University College, British Columbia, 3. Functional Groups with Prefixes Only. Canada II. Alkoxyalkanes (Ethers). Click to reveal examples of naming ethers. (Contains many examples.) Note: Your browser must have Java installed. If you have problems, go to the top of the page at http://www.molecularmodels.ca/nomenclature/index-2.htm and follow directions to manipulate structure and if necessary download JAVA. If you have problems contact me. c. http://www.acdlabs.com/iupac/nomenclature Developed by Advanced Chemistry Development Laboratories (Gives detailed rules for nomenclature.) Recommendations 1993 R-5 Applications to Specific Classes of Compounds R-5.5 Hydroxy Compounds, their Derivatives and Analogues R-5.5.4 Ethers and chalcogen analogues (consider only ethers) R-5.5.4.1 Substitutive names R-5.5.4.2 Functional class names 3. Applications a. Name the following: O O O Cl b. Draw structural formulas for the following compounds: Br O 9 Acid-Base Rxns & Energy Relationships-4 Propyl cyclopentyl ether 2,5-dimethoxyhexane 1-bromo-1-propoxycycloheptane dicyclobutyl ether