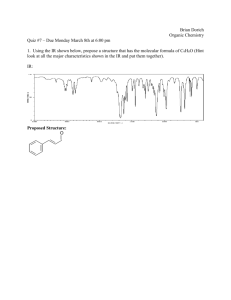
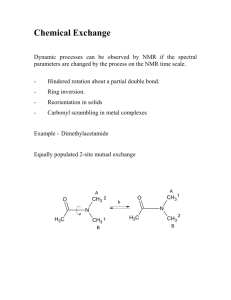
Chemical Reaction Kinetics Exercises
advertisement
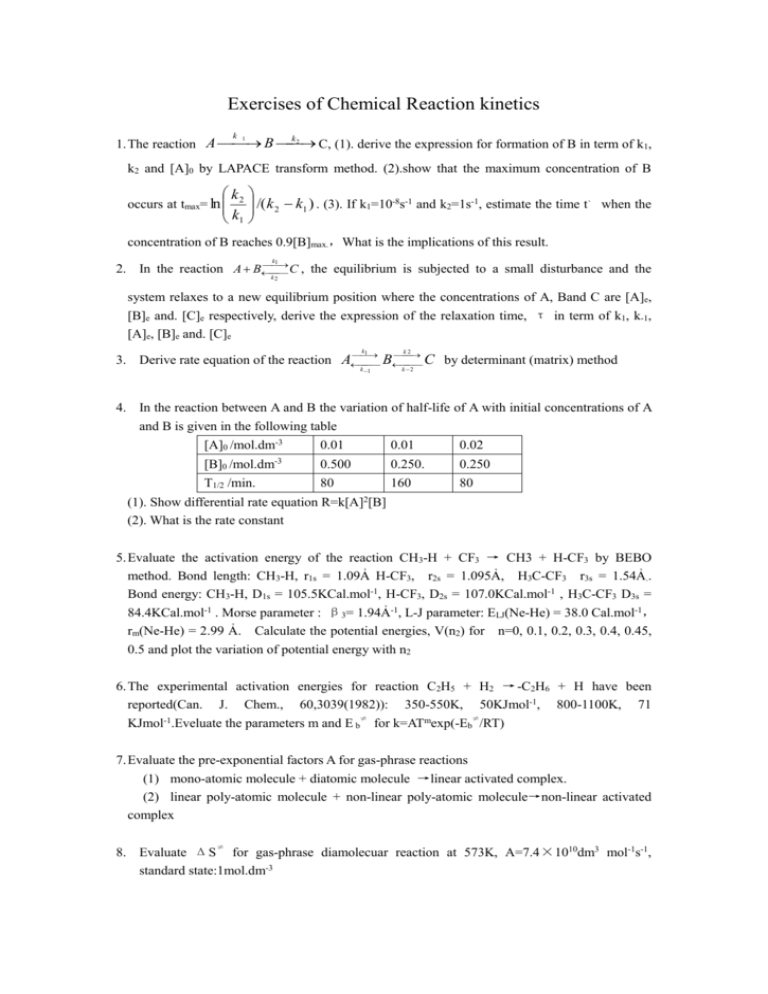
Exercises of Chemical Reaction kinetics k 2 1. The reaction A B C, (1). derive the expression for formation of B in term of k1, k 1 k2 and [A]0 by LAPACE transform method. (2).show that the maximum concentration of B k2 /( k 2 k1 ) . (3). If k1=10-8s-1 and k2=1s-1, estimate the time t、 when the k1 occurs at tmax= ln concentration of B reaches 0.9[B]max.,What is the implications of this result. k1 2. In the reaction A B C , the equilibrium is subjected to a small disturbance and the k2 system relaxes to a new equilibrium position where the concentrations of A, Band C are [A]e, [B]e and. [C]e respectively, derive the expression of the relaxation time, τ in term of k1, k-1, [A]e, [B]e and. [C]e k 1 k2 k 1 k 2 3. Derive rate equation of the reaction A B C by determinant (matrix) method 4. In the reaction between A and B the variation of half-life of A with initial concentrations of A and B is given in the following table [A]0 /mol.dm-3 [B]0 /mol.dm-3 T1/2 /min. (1). Show differential rate equation (2). What is the rate constant 0.01 0.01 0.02 0.500 0.250. 0.250 80 160 80 R=k[A]2[B] 5. Evaluate the activation energy of the reaction CH3-H + CF3 → CH3 + H-CF3 by BEBO method. Bond length: CH3-H, r1s = 1.09Ả H-CF3, r2s = 1.095Ả, H3C-CF3 r3s = 1.54Ả.. Bond energy: CH3-H, D1s = 105.5KCal.mol-1, H-CF3, D2s = 107.0KCal.mol-1 , H3C-CF3 D3s = 84.4KCal.mol-1 . Morse parameter : β3= 1.94Ả-1, L-J parameter: ELJ(Ne-He) = 38.0 Cal.mol-1, rm(Ne-He) = 2.99 Ả. Calculate the potential energies, V(n2) for n=0, 0.1, 0.2, 0.3, 0.4, 0.45, 0.5 and plot the variation of potential energy with n2 6. The experimental activation energies for reaction C2H5 + H2 → -C2H6 + H have been reported(Can. J. Chem., 60,3039(1982)): 350-550K, 50KJmol-1, 800-1100K, 71 ≠ ≠ KJmol-1.Eveluate the parameters m and E b for k=ATmexp(-Eb /RT) 7. Evaluate the pre-exponential factors A for gas-phrase reactions (1) mono-atomic molecule + diatomic molecule →linear activated complex. (2) linear poly-atomic molecule + non-linear poly-atomic molecule→non-linear activated complex ≠ 8. Evaluate ΔS for gas-phrase diamolecuar reaction at 573K, A=7.4×1010dm3 mol-1s-1, standard state:1mol.dm-3 9. Evaluate k2/k-1 when ku=80%k∞ and [M]+10-4mol.L-1for unimolecular reaction. The k1 * Lindemann’s mechanism: A M A M k 1 , k2 A* P 10. Rate constants for gas-phrase dimerization of cyclic pentylenes and the bimer decomposition at 373K are as follows: Dimerized: k2=106.1exp(-16700x4.184/RT)dm3mol-1s-1 Decomposed: k1=1013.1exp(-35000x4.184/RT)s-1 ≠ Evaluate ΔS for forward and back reaction. What activated complex is shown? 11. The expression of k for the unimolecular reaction at 500K is k =5.2×1014exp(43700× 4.184//RT)s-1. how many degees ,S of vibrational freedom are there at least if ku is still equal to k∞ at P=150mmHg? Derivation with RRK theory. The cross sectional area σAA= (1.0nm)2, molecular weight, mA=160 12. Derivate the rate constant of unimolecular reaction at high pressure limit with kRRKM, theory. 13. The decomposition reaction of acetaldehyde is CH3CHO → CH4 + CO. The reaction mechanism is listed below: Initial CH3CHO → CH3•+ CHO• Propagation CH3•+ CH3CHO → CH4 + CH3• + CO Termination 2CH3• → C2H6 (1). Determine the production rate of CH4 with the steady state approximation for the CH3 intermediate. (2). Evaluate the activation energy for the initial step., when the activation energies for the overall reaction, the propagation step and termination step are given, Eexp=192.5, Ep=33.5, ET=0KJmol-1, respectively. 14. Suggest plausible mechanism for the thermal decomposition of acetone 1.CH3COCH3 → CH3CO• + CH3• 84Kcalmol-1 2. CH3CO• → CH3• + CO 16 3. CH3• + CH3COCH3 → CH4 + CH2COCH3• 15 4. CH2COCH3• → CH3• + CH2CO 48 5. CH3• + CH2COCH3 → C2H5COCH3 5 (1). Derive the expression for the thermal decomposition rate equation of acetone in terms of ki(i=1-5) and [CH3COCH3] (2). Estimate the activation energy of the overall reaction. 15. The mechanism for the reaction H2 + Br2→ 2HBr is as follows: Br2 → 2Br• E0=192KJmol-1 Br• + H2 → HBr + H• E1=72KJmol-1 H• + Br2 → HBr + Br• E2=5KJmol-1 H• + HBr →H2 + Br• E-1=5KJmol-1 2Br• → Br2 ET=0KJmol-1 Estimate the activation energy of the overall reaction Eexp. when (1). In the initial (2). In the end 16. (1) Derive the expression for the rate equation of the overall reaction H2 + I2→ 2HI. The mechanism : k I2 1 k 1 I• + H2 2I• k2 k 2 fast H2I• k3 H2I• + I• 2HI fast slow (2). What is the expression if the mechanism: k0 I2 2I• k1 I• + H2 HI + H• k2 H• + I2 HI + I• kT 2I• I2