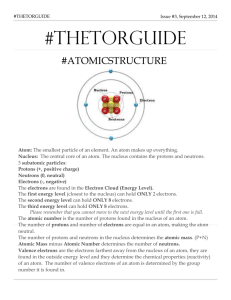
Atomic Structure
advertisement
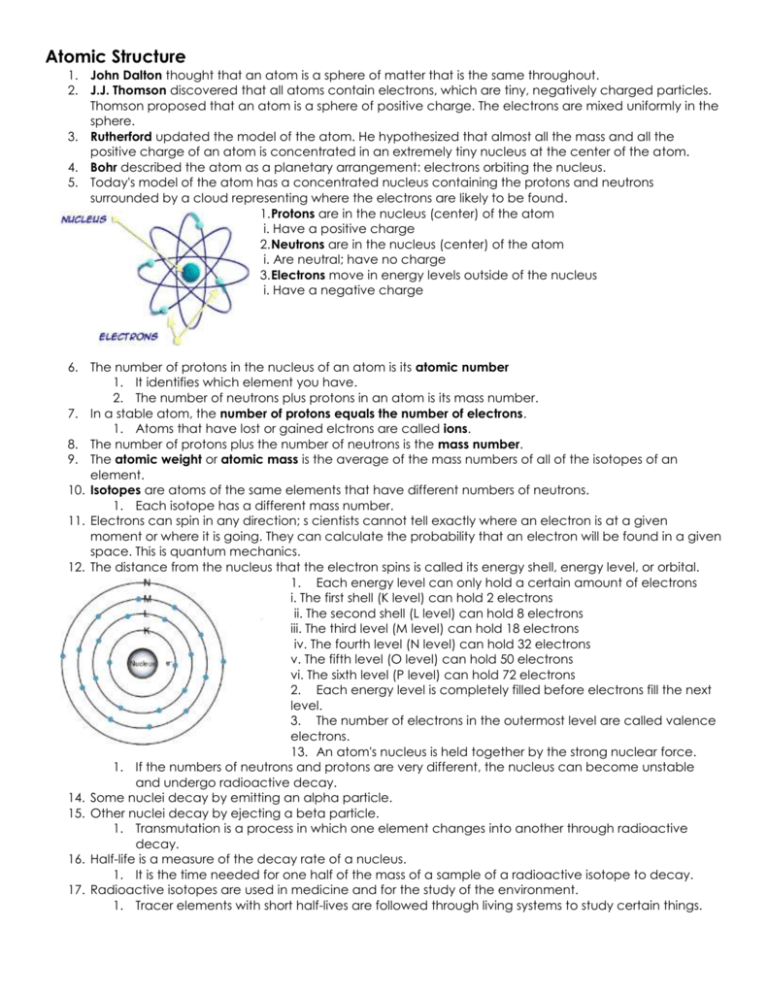
Atomic Structure 1. John Dalton thought that an atom is a sphere of matter that is the same throughout. 2. J.J. Thomson discovered that all atoms contain electrons, which are tiny, negatively charged particles. Thomson proposed that an atom is a sphere of positive charge. The electrons are mixed uniformly in the sphere. 3. Rutherford updated the model of the atom. He hypothesized that almost all the mass and all the positive charge of an atom is concentrated in an extremely tiny nucleus at the center of the atom. 4. Bohr described the atom as a planetary arrangement: electrons orbiting the nucleus. 5. Today's model of the atom has a concentrated nucleus containing the protons and neutrons surrounded by a cloud representing where the electrons are likely to be found. 1. Protons are in the nucleus (center) of the atom i. Have a positive charge 2. Neutrons are in the nucleus (center) of the atom i. Are neutral; have no charge 3. Electrons move in energy levels outside of the nucleus i. Have a negative charge 6. The number of protons in the nucleus of an atom is its atomic number 1. It identifies which element you have. 2. The number of neutrons plus protons in an atom is its mass number. 7. In a stable atom, the number of protons equals the number of electrons. 1. Atoms that have lost or gained elctrons are called ions. 8. The number of protons plus the number of neutrons is the mass number. 9. The atomic weight or atomic mass is the average of the mass numbers of all of the isotopes of an element. 10. Isotopes are atoms of the same elements that have different numbers of neutrons. 1. Each isotope has a different mass number. 11. Electrons can spin in any direction; s cientists cannot tell exactly where an electron is at a given moment or where it is going. They can calculate the probability that an electron will be found in a given space. This is quantum mechanics. 12. The distance from the nucleus that the electron spins is called its energy shell, energy level, or orbital. 1. Each energy level can only hold a certain amount of electrons i. The first shell (K level) can hold 2 electrons ii. The second shell (L level) can hold 8 electrons iii. The third level (M level) can hold 18 electrons iv. The fourth level (N level) can hold 32 electrons v. The fifth level (O level) can hold 50 electrons vi. The sixth level (P level) can hold 72 electrons 2. Each energy level is completely filled before electrons fill the next level. 3. The number of electrons in the outermost level are called valence electrons. 13. An atom's nucleus is held together by the strong nuclear force. 1. If the numbers of neutrons and protons are very different, the nucleus can become unstable and undergo radioactive decay. 14. Some nuclei decay by emitting an alpha particle. 15. Other nuclei decay by ejecting a beta particle. 1. Transmutation is a process in which one element changes into another through radioactive decay. 16. Half-life is a measure of the decay rate of a nucleus. 1. It is the time needed for one half of the mass of a sample of a radioactive isotope to decay. 17. Radioactive isotopes are used in medicine and for the study of the environment. 1. Tracer elements with short half-lives are followed through living systems to study certain things.