Powerpoint (PPT - 1 MB)
advertisement

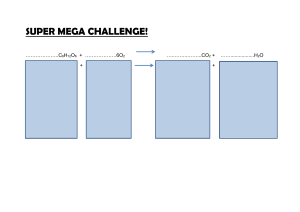
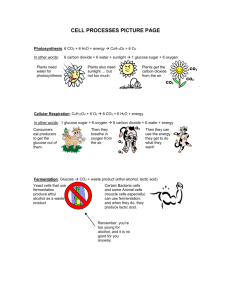
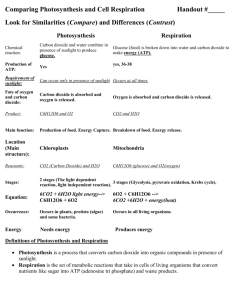
Introducing the Geochemical Cycles (it is necessary to understand & use these if you want to design a Mars Colony or any ecosystem…) Water cycle Carbon cycle Energy NON-cycle Part II – the Carbon Cycle: Most of the carbon on earth is locked up in the crust in limestone rocks: CaCO3 (PUT THIS IN YOUR NOTES!) Some of the rest is in fossil fuels: coal, crude oil and natural gas. Coal = carbon Methane = CH4 (PUT IN Some is in the atmosphere as CO2: (PUT IN NOTES) A lot is dissolved in seawater as carbonate (CO32-), bicarbonate (HCO3-), carbonic acid (H2CO3) and carbon dioxide (CO2): (PUT IN NOTES) And of course some is in living & dead biological tissue (Biomass): (NOTES) PHOTOSYNTHESIS (plants and Algae): 6CO2 + 6H2O (sunlight) C6H12O6 + 6O2 RESPIRATION (all life) C6H12O6 + 6O2 6CO2 + 6H2O (+ energy!) Where: C6H12O6 = sugar O2 = oxygen CO2 = carbon dioxide H2O = water (PUT THIS IN NOTES) Draw in some ball-and-stick molecules… carbon dioxide carbonate bicarbonate carbonic acid calcium carbonate water (PUT THIS IN NOTES) Carbon Cycle getting started… (don’t put this in your notes!) CO2 Limestone CaCO3 CO2 The main thing is to present it as a CYCLE That means every place with carbon has at least one arrow coming in and one arrow going out!