Review-nomenclature-reactions answer key
advertisement

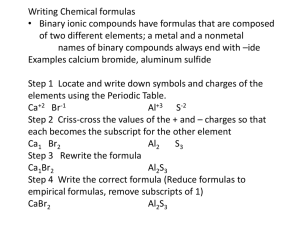
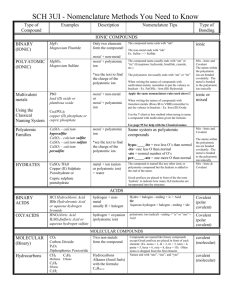
ANSWER KEY Station One: Types of Compounds Type I: Ionic Compound - Has a metal NOT from the transition metal section of the PT and a nonmetal/polyatomic ion. Does NOT use Roman numerals in the name Type II: Ionic Compound - Has a metal from the transition metal section of the PT and a nonmetal/polyatomic ion. Uses Roman numerals in the name Type III: Covalent Compound - Made of 2 nonmetals. Uses prefixes in the name Type Acids: - H is ALWAYS the first element in the compound The name always says acid in it! If there is NO oxygen in the formula, it’s a binary acid and you HAVE to use the prefix hydro- in the name. If there is oxygen in the formula, it’s an oxyacid and it will end in either –ic acid (comes from –ate) or –ous acid (comes from –ite). Directions: Identify the type of compound for each substance. 1. N2O3 Type III 2. H2CO3 Type Acid (oxyacid) 3. Aluminum sulfate Type I 4. Cobalt (III) nitride Type II 5. HBr Type Acid (binary acid) 6. Tetraphosphorous decoxide Type III 7. K3PO4 Type I 8. MnF2 Type II 9. Strontium chloride Type I 10. Au2(SO3)3 Type II Station 2: Writing Formulas Type I: Ionic Compounds - Write the symbol and charge of the metal, use the periodic table to determine the charge. - Write the symbol and charge of the nonmetal/polyatomic ion. - Criss-cross the charges. o If you cross a number to a polyatomic ion, put the ion in parentheses first. Type II: Ionic Compounds - Write the symbol and charge of the metal, the Roman Numerals tell you the charge for the metal - Write the symbol and charge of the nonmetal/polyatomic ion. - Criss-cross the charges. o If you cross a number to a polyatomic ion, put the ion in parentheses first. Type III: Covalent Compounds - Write the symbol for the first element; change the prefix to a subscript. - Do the same thing for the 2nd element. Directions: Write the symbol for the following compounds. 1. Magnesium bromide MgBr2 2. Iron (III) sulfide Fe2S3 3. Diphosphorous pentoxide P2O5 4. Potassium nitrite KNO3 5. Copper (II) hydroxide Cu(OH)2 6. Sulfur trioxide SO3 7. Barium phosphate Ba3(PO4)2 8. Vanadium (V) chlorite V(ClO2)5 9. Xenon hexafluoride XeF6 Station 3: Naming compounds Type I: Ionic Compounds - Write the name of the metal - Write the name of the nonmetal, change the ending to –ide. OR - Write the name of the polyatomic ion. Type II: Ionic Compounds - Write the name of the metal. - You need to write the original charge of the metal as a Roman Numeral. You will have to un-criss-cross the charges to do so. - Write the name of the nonmetal, change the ending to –ide. OR - Write the name of the polyatomic ion. Type III: Covalent Compounds - Write the name of the first element. - Change the subscript to a prefix. - Do the same for the 2nd element, just remember that the 2nd element must end in –ide. Directions: Write the names of the following compounds. 1. Na2O sodium oxide 2. CrI3 chromium (III) iodide 3. H2O dihydrogen monoxide 4. CaCO3 calcium carbonate 5. Pd2(SO3)3 palladium (III) sulfate 6. IF7 iodine heptafluoride 7. Al(NO3)3 aluminum nitrate 8. AgNO2 silver (I) nitrite 9. CCl4 carbon tetrachloride Station 4: Acids Formulas - Is hydro- part of the name? Then there is NO oxygen in the formula! o Write H+. o Write the 2nd element’s symbol and charge. o Criss-cross the charges. - If there is NO hydro, does it end in –ic acid or –ous acid? o If it ends in –ic acid… Write H+. Write the symbol and charge of the polyatomic ion that ends in –ate. Criss-cross charges. o If it ends in –ous acid… Write H+. Write the symbol and charge of the polyatomic ion that ends in –ite. Criss-cross charges. Names - If there is NO oxygen in the formula, it’s a binary acid. o Write the prefix hydroo Write the first part of the 2nd element o End with –ic acid. - If there is oxygen in the formula, check out the polyatomic ion. o Does the name of the polyatomic ion end in –ate? Change –ate to –ic acid. o Does the name of the polyatomic ion end in –ite? Change –ite to –ous acid. Directions: Write the formula for these acids. 1. Hydrofluoric acid HF 2. Nitric acid HNO3 3. Hydrosulfuric acid H2 S 4. Nitrous acid HNO2 Directions: Write the name for these acids. 5. H3PO4 phosphoric acid 6. H3N hydronitric acid 7. HI hydroiodic acid 8. H2SO3 sulfurous acid Station 5: Balancing Chemical Equations Use coefficients to make sure that you have the same number of atoms of each element on the left and right side of the equation! Remember, a coefficient multiples through an entire formula! Directions: Balance the equations. 1. 2H2O(l) + Ba(s) → H2(g) + Ba(OH)2(aq) 2. 3Na2SO4(aq) + 2FePO4(aq) → 2Na3PO4(aq) + Fe2(SO4)3(s) 3. 2H2O2(aq) → 2H2O(l) + O2(g) 4. 2HBr(aq) + Ca(OH)2(aq) → CaBr2(aq) + 2H2O(l) 5. C5H12(l) + 8O2(g) → 5CO2(g) + 6H2O(g) Station 6: Types of Reaction - Synthesis o Reactant 1 + Reactant 2 → Product o Ex. H2 + Cl2 → 2HCl - Desomposition o Reactant → Product 1 + Product 2 o Ex. 2 NaCl → 2Na + Cl2 - Single-replacement o Element 1 + Compound 1 → Element 2 + Compound 2 o 2Li + MgSO4 → Mg + Li2SO4 - Double-replacement o 2 compounds switch ions o NaNO3 + KBr → NaBr + KNO3 - Combustion o Hydrocarbon + Oxygen → Carbon dioxide + Water o CH4 + 2O2 → CO2 + 2H2O Directions: Identify the type of reaction for each equation. 1. H2O(l) + Ba(s) → H2(g) + Ba(OH)2(aq) single-replacement 2. Na2SO4(aq) + FePO4(aq) → Na3PO4(aq) + Fe2(SO4)3(s) double-replacement 3. H2O2(aq) → H2O(l) + O2(g) decomposition 4. HBr(aq) + Ca(OH)2(aq) → CaBr2(aq) + H2O(l) double-replacement 5. C5H12(l) + O2(g) → CO2(g) + H2O(g) combustion 6. CaCO3(s) → CaO(s) + CO2(g) decomposition 7. C2H5OH(l) + O2(g) → CO2(g) + H2O(g) combustion 8. Na(s) + Al(OH)3(aq) → Al(s) + NaOH(aq) single-replacement 9. Mg(s) + N2(g) → Mg3N2(s) synthesis 10. Pb(NO3)3 + H2SO4(aq) → PbSO4(s) + HNO3(aq) double-replacement