Rules for Naming Ionic Compounds
advertisement

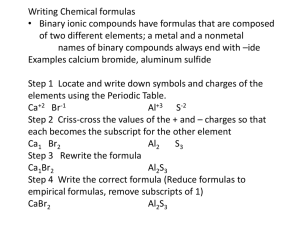
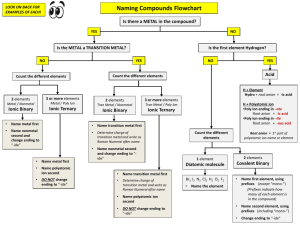
Rules for Naming Ionic Compounds I. Four different types of ionic compounds A. Ionic compounds metal and nonmetal 1. Metal of Family IA, IIA, IIIA, Zn, Cd, Ag and a nonmetal a. Name the metal and then name the nonmetal changing the ending to the -ide suffix. b. Zinc, Cadmium and Silver always have the same charges so they follow the same rule as above. (Zn2+, Cd2+, Ag+) c. Examples: NaCl K2S Mg3N2 ZnO = = = = sodium chloride potassium sulfide magnesium nitride zinc oxide 2. Metal of Families IVA, VA and Transition metals and a nonmetal a. These metals can have more than one charge. A roman numeral is added to the name to indicate the positive charge of the metal. The nonmetal is named changing the ending to the -ide suffix. b. Examples: FeO = iron (II) oxide Fe2O3 = iron (III) oxide CuCl2 = copper (II) chloride Nonmetal Root names bor- carb- nitr- ox- silic- phospharsen- sulfselentellur- hydrfluorchlorbromiodastat- B. Ionic compounds metal and polyatomic ion. Polyatomic ion - tightly bound group of atoms that behaves as a unit and carries a charge that is usually negative. 3. Metal of Family IA, IIA, IIIA, Zn, Cd, Ag and a polyatomic ion a. Name the metal and then name the polyatomic ion b. Examples: Al2(CO3)3 Na2SO4 AgClO3 (NH4)2SO4 = = = = aluminum carbonate sodium sulfate silver chlorate ammonium sulphate NH4+ Ammonium Ion is the exception 4. Metal of Families IVA, VA and Transition metals and a nonmetal a. Name the metal, use a Roman numeral to indicate the positive charge of the metal and name the polyatomic ion. b. Examples: Cu(NO3)2 = copper (II) nitrate MnSO3 = manganese (II) sulfite SnCrO4 = tin (II) dichromate Rules for Naming Covalent Compounds Naming Compounds Containing Two Nonmetals: when two nonmetals are combined in a compound, it is important that the name indicate the number of atoms of each element that are present because more than one compound can exist containing the same two nonmetals. For example, nitrogen and oxygen form a number of different compounds with chemical formulas such as: NO, NO2, N2O4, etc. It is essential that the names of these compounds be different. A system is used in which Greek number prefixes are employed to indicate how many atom of each element are present. You will need to learn the following number prefixes if you do not already know them. Greek Prefixes: 1 (mono) 2 di- 3 tri- 4 5 tetra- penta- 6 hexa- 7 hepta- 8 octa- 9 nona- 10 deca- If there is only one atom of the first nonmetal, the prefix, mono, is not used, but in all other cases, the number prefix is attached to the name of the nonmetal to indicate how many atoms of each element are present. Again, because the compound contains only two elements, the root of the name of the second element is given an -ide ending. Writing Covalent Formulas carbon dioxide (mono) carbon di - ox -ide C1 O2 CO2 (note: mono is implied for the first element, it is not called mono- ) carbon monoxide (mono) carbon dinitrogen pentoxide mono - ox -ide C1 O1 di - nitrogen penta - ox -ide N2 O5 CO N2O5 Naming Covalent Compounds Greek prefix (Nonmetal name) + Greek prefix (Nonmetal root name) -ide CO2 1-carbon 2-ox(ygen)-ide (mono)carbon di - ox - ide carbon dioxide (note: mono is implied for the first element, it is not called mono- ) CO 1-carbon 1-ox(ygen)-ide (mono)carbon mono -ox -ide carbon N2O5 2-nitrogen 5-ox(ygen)-ide di -nitrogen penta -ox -ide Other Examples: SO3 = sulfur trioxide P4O10 = tetraphosphorus decaoxide H2O = dihydrogen monoxide monoxide dinitrogen pentaoxide Rules for Naming Acids Hydrogen and another nonmetal - all acids contain hydrogen as the first element and are given acid names when in aqueous solution which is denoted by (aq) following the formula. 1. If the formula is followed by (g) denoting a gaseous state, the compound is named in the same way as Rule A-1 (See ionic naming rules page 1) Examples: HCl (g) = hydrogen chloride H2Te (g) = hydrogen telluride Note: No Greek Prefixes are used when H is the cation 2. If the formula is followed by (aq) denoting an acid solution, add a hydro- prefix to the root of the second nonmetal name and add an -ic suffix then the word acid H2S (aq) = hydrosulfuric acid Examples: HCl (aq) = hydrochloric acid NOTE: The acid with only two different atoms has a longer name. Hydrogen and a polyatomic ion - all acids contain hydrogen as the first element and are given acid names . 1. Polyatomic ions ending in -ite a. Add an -ous suffix to the polyatomic ion name and add the word acid. b. Examples: HNO2 = (hydrogen nitrite) = nitrous acid H2SO3 = (hydrogen sulfite) = sulfurous acid HClO = (hydrogen hypochlorite) = hypochlorous acid c. Mnemonic – Last n-ite, I saw a m-ous-e. 2. Polyatomic ions ending in -ate a. Add an -ic suffix to the polyatomic ion name and add the word acid. b. Examples: H2CO3 = (hydrogen carbonate) = carbonic acid H3PO4 = (hydrogen phosphate) = phosphoric acid HBrO4 = (hydrogen perbromate) = perbromic acid c. Mnemonic – I -ate a bug -ic-k NOTE: The acid with three or more different atoms has a shorter name.