Test Review
advertisement

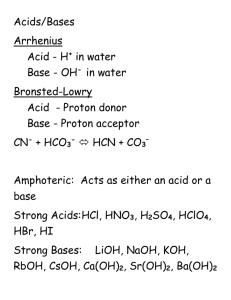
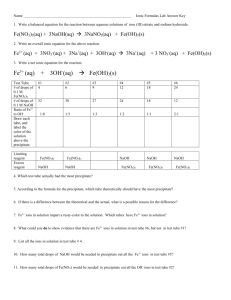
Chemistry 11 Test #4 Topics: For this test you should know… 1. Solution Properties Polarity and miscibility Factors affecting solubility 2. Solution Concentration Calculating concentration Preparing solutions 3. Solubility Solubility rules Net ionic equations Solubility curves 4. Solution Stoichiometry Solution stoichiometry calculations 5. Acids/Bases Acid/base theories pH scale and calculations Titration calculations for acids/bases Review Questions: Solution Properties 1. Say whether each of the following compounds would dissolve/mix with water. a. Ethanol, CH3CH2OH (Yes) b. Carbon tetrachloride, CCl4 (No) c. Potassium chloride, KCl (Yes) 2. Describe how each of the following factors affect solubility of ionic compounds in water: a. Charge on ions b. Temperature c. Size of ions Solution Concentration 3. Determine the mass % (m/m) of a NaCl solution if 58.5 grams of NaCl was dissolved in 50 ml of water (assume the density of water to be 1.0 g/ml). (54%) 4. If we wished to prepare 400 ml of a 10% by mass (m/m) NaCl solution what mass of NaCl would we use? Density of the solution is 1.05 g/ml. (42g) 5. How would you prepare 1000 ml of a 5.0% (v/v) Glycol solution in water? (vol glycol = 50 mL) 6. Robitussin cough medicine contains the following ingredients per teaspoonful (5.0 ml): 100 mg guaifenesin and 10 mg dextromethorphan hydrobromide. What is the percentage concentration (m/v) for each ingredient? (2%, .2%) 7. If an adrenaline rush can be experienced when your blood contains only 5.4 ppm of the hormone, what total mass of adrenaline is required in the blood of a person who has 6.5 liters of blood total for the hormone to kick in? (assume density of blood = 1.00g/mL) (0.035g) 8. Calculate the molarity of each of the following: a. A 5.623-g sample of NaHCO3 is dissolved in enough water to make 250.0 mL of solution. (.2677 M) b. A 184.6-mg sample of K2Cr2O7 is dissolved in enough water to make 500.0 mL of solution. (0.00126 M) 9. Calculate the concentration of all ions present in each of the following solutions of strong electrolytes. a. 0.15 M CaCl2 (Ca: 0.15 M; Cl: 0.30 M) b. 0.26 M Al(NO3)2 (Al: 0.26 M; NO3: 0.52 M) 10. What volume of a 0.100 M solution of NaHCO3 contains 0.350 g of NaHCO3? (0.0417 L) 11. Describe how you would prepare 2.00 L of each of the following: a. 0.250 M NaOH from solid NaOH (mass = 20.0g) b. 0.250 M NaOH from 1.00 M NaOH stock solution. (Vol stock = 0.500L) 12. A solution is prepared by dissolving 10.8 g ammonium sulfate in enough water to make 100.0 mL of stock solution. A 10.00 mL sample of this stock solution is added to 50.00 mL of water. Calculate the concentration of ammonium sulfate in the final solution. (0.158 M) Solubility 13. When the following solutions are mixed together, what precipitate (if any) will form? a. FeSO4(aq) + KCl(aq) (None) b. Al(NO3)3(aq) + Ba(OH)2(aq) (Al(OH)3) 14. Write net ionic equations for each of the following: a. AgNO3(aq) + KI(aq) b. CuSO4(aq) + Na2S(aq) Solution Stoichiometry 15. What mass of NaCl is required to precipitate all the silver ions from 50.0 mL of 0.0500 M solution of AgNO3? (0.146g) 16. What mass of solid aluminum hydroxide is produced when 50.0 mL of 0.200 M Al(NO3)3 is added to 200.0 mL of 0.100 M KOH? (0.520 g) Acids/Bases 17. What are the conjugate pairs for the following: a. HNO3(g) + H2O(l) b. NH3(g) + H2O(l) 18. Calculate the pH of the following: a. Solution with [H3O+] = 4.0 x 10-8 mol/L (7.40) b. 0.100 M HCl (1.000) 19. What volume of each of the following acids will react completely with 50.00 mL of 0.200 M NaOH? a. 0.100 M HCl (100. mL) b. 0.150 M HNO3 (66.7 mL) c. 0.100 M H2SO4 (50.0 mL) 20. A 25.00-mL sample of hydrochloric acid requires 24.16 mL of 0.106 M sodium hydroxide to reach the equivalence point. What is the concentration of the original hydrochloric acid sample? (0.102 M) p. 487-488 #1-3, 7, 10, 12, 14, 15, 17-24, 26, 30, 33