Shao Exam 2 Practice Problems
advertisement

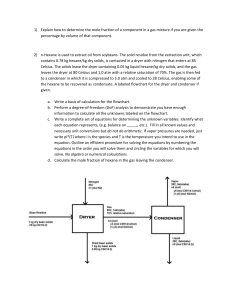
Shao Exam 2 Example Problems and Study Guide Supplemental Instruction Iowa State University Leader: Course: Instructor: Date: Wheaton CH E 210 Dr. Shao 11/10/14 Non-Ideal Equations of State (Lecture 23) Be able to use the Virial, Van der Waals, and Soave-Redlich-Kwong (SRK) equations of state when the ideal gas law does not apply. Know when the ideal gas law is applicable. Calculate the following using any or all of the three non-idea equation of state presented a) 𝑁2 : 𝑛 = 10 𝑚𝑜𝑙𝑒𝑠, 𝑉 = 40 𝐿, 𝑇 = 800 𝐾 b) 𝑊𝑎𝑡𝑒𝑟: 𝑛 = 1 𝑚𝑜𝑙𝑒, 𝑉 = 10 𝐿, 𝑇 = 1000 𝐾 Compressibility Factor (Lecture 24) Be able to use the compressibility factor equation of state for systems where the ideal gas law does not apply. Calculate the following: a) 𝐻2 : 𝑃 = 41.6 𝑎𝑡𝑚, 𝑇 = 49.56 𝐾, 𝑛 = 100 𝑚𝑜𝑙𝑒𝑠, 𝑓𝑖𝑛𝑑 𝑍, 𝑉 b) 𝐶ℎ𝑙𝑜𝑟𝑜𝑓𝑜𝑟𝑚: 𝑍 = 0.8, 𝑃 = 27 𝑎𝑡𝑚, 𝐹𝑖𝑛𝑑 𝑇, 𝑉̂ 𝐿 c) 𝐶ℎ𝑙𝑜𝑟𝑜𝑏𝑒𝑛𝑧𝑒𝑛𝑒: 𝑉̂ = 2.91 , 𝑇 = 2213.4 𝐾, 𝑓𝑖𝑛𝑑 𝑍, 𝑃 𝑚𝑜𝑙 d) 𝐴𝑐𝑒𝑡𝑜𝑛𝑒: 𝑇 = 508 𝐾, 𝑃 = 1410 𝑎𝑡𝑚, 𝑓𝑖𝑛𝑑 𝑍, 𝑉̂ Pseudocritical Temperature and Pressure, Kay’s Rule (Lecture 25) Be able to use the compressibility factor equation of state for gas mixtures using Kay’s Rule. Calculate the following: a) 𝐴𝑖𝑟: 𝑃 = 18.45 𝑎𝑡𝑚, 𝑇 = 145.33 𝐾, 𝑓𝑖𝑛𝑑 𝑍, 𝑉 b) 𝐸𝑞𝑢𝑖𝑚𝑜𝑙𝑎𝑟 𝑛 − 𝐵𝑢𝑡𝑎𝑛𝑒 𝑎𝑛𝑑 𝐵𝑒𝑛𝑧𝑒𝑛𝑒: 𝑍 = 0.8, 𝑉̂𝑖𝑑𝑒𝑎𝑙 = 1, 𝑓𝑖𝑛𝑑 𝑃, 𝑇 Reading a Phase Diagram (Lecture 26) Be comfortable labeling the parts of a phase diagram. If you are given a path on a phase diagram (such as figure 6.1-1 on page 241), be able to describe the state of the species at each point. The graph is not precise, so for temperatures and pressures use the closest marked value. a) solid phase b) gas phase c) liquid phase d) vapor liquid equilibrium e) gas liquid equilibrium f) gas solid equilibrium g) triple point h) critical point i) supercritical fluid j) sublimation temperature at k) boiling temperature at 5 l) melting temperature at 5 1 atm atm atm 1060 Hixson-Lied Student Success Center 515-294-6624 sistaff@iastate.edu http://www.si.iastate.edu Cox Chart (Lecture 27) Be familiar with how to read a Cox Chart given species and temperature Find the vapor pressure of the following a) Methyl Chloride at 0𝑜 𝐹 b) Nitrobenzene at 400𝑜 𝐹 c) Acetone at 100𝑜 𝐹 Vapor Pressure, Humidity, Degrees of Superheat (Lecture 28) You should be comfortable calculating 𝑇, 𝑃, 𝑦𝐴 , 𝑝𝐴 , 𝐷𝑒𝑤 𝑃𝑜𝑖𝑛𝑡, 𝐷𝑒𝑔𝑟𝑒𝑒𝑠 𝑜𝑓 𝑆𝑢𝑝𝑒𝑟ℎ𝑒𝑎𝑡, 𝑎𝑛𝑑 ℎ𝑢𝑚𝑖𝑑𝑖𝑡𝑦.given any of the three quantities for non-equilibrium systems, or two quantities for equilibrium: a) Air at 1 𝑎𝑡𝑚 and 70𝑜 𝐶 has a partial pressure of water of 142.60 𝑚𝑚 𝐻𝑔. Calculate the mole fraction of water in the air, the dew point, the degrees of superheating, and relative humidity of the air. b) 1 L container half filled with liquid n-hexane (while the rest of the volume is filled with dry air at 1 atm), is left in a chemical storage cabinet at room temperature (25𝑜 𝐶), and the system is allowed to reach equilibrium. Calculate the mole fraction of hexane in the air, dew point, degrees of superheating, partial pressure, and molal saturation of n-hexane. Example System using Raoult’s Law (single and multiple condensable species), Humidity, Degrees of Superheating (Lectures 28 and 29) Air Feed 1 atm, 30 C, 10 L/min 80% relative humidity Liquid Water Dehumidifier Dried Air 30 C, Degrees of superheating = 25 C Liquid hexane input Furnace output, Gas phase, 500 C Furnace 80% Conversion 10% Excess Air All product gases except water and hexane 500 C Seperator Water and Hexane Water and Hexane Vapor 30 C 1 atm Condensor Water and Hexane Liquid 30 C Gibbs Phase Rule (Lecture 30) Given a number of species and phases (and possibly already defined intensive variables), determine the number of variables needed yet to solve the system. Gibbs Phase rule: 2 + 𝑐 − Π = 𝐷𝐹 Determine the number of intensive variables that must be solved for to the system to be fully defined? What intensive variables may be defined to reduce the degrees of freedom to zero? a) A liquid phase mix of acetone, water, and MIBK at a given pressure and temperature b) A liquid-vapor mixture of hexane and benzene c) Carbon dioxide present in solid, liquid, and vapor phases d) Pure liquid cloroform Triangular Phase Diagrams (Lecture 31) Given three partially misciable species and their wt%, be able to determine the number of liquid phases and composition of each phase. Be able to identify the major parts of a triangular phase diagram. A steam is an even wt% mixture of Acetone and water, and is fed at a rate of 100 kg/h to a mixing/settling unit which adds 50 kg/h of MIBK. a) Give the composition of the each product phase resulting from the first mixing/settling unit. b) Another mixing/settling step is added to further refine the water (to a value of 80 wt%). If the water rich phase from part a is fed to this step, what is the rate at which MIBK must be added to achieve the desired wt% in the water rich phase product?