exam 2 review
1) Explain how to determine the mole fraction of a component in a gas mixture if you are given the percentage by volume of that component.
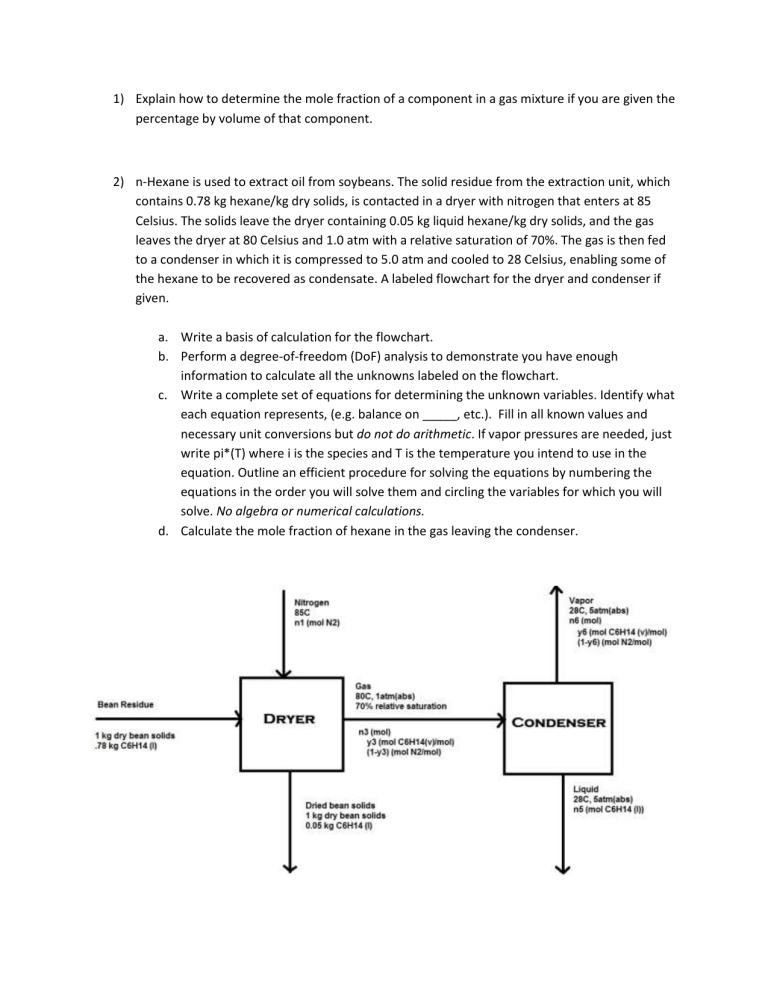
2) n-Hexane is used to extract oil from soybeans. The solid residue from the extraction unit, which contains 0.78 kg hexane/kg dry solids, is contacted in a dryer with nitrogen that enters at 85
Celsius. The solids leave the dryer containing 0.05 kg liquid hexane/kg dry solids, and the gas leaves the dryer at 80 Celsius and 1.0 atm with a relative saturation of 70%. The gas is then fed to a condenser in which it is compressed to 5.0 atm and cooled to 28 Celsius, enabling some of the hexane to be recovered as condensate. A labeled flowchart for the dryer and condenser if given. a.
Write a basis of calculation for the flowchart. b.
Perform a degree-of-freedom (DoF) analysis to demonstrate you have enough information to calculate all the unknowns labeled on the flowchart. c.
Write a complete set of equations for determining the unknown variables. Identify what each equation represents, (e.g. balance on _____, etc.). Fill in all known values and necessary unit conversions but do not do arithmetic. If vapor pressures are needed, just write pi*(T) where i is the species and T is the temperature you intend to use in the equation. Outline an efficient procedure for solving the equations by numbering the equations in the order you will solve them and circling the variables for which you will solve. No algebra or numerical calculations. d.
Calculate the mole fraction of hexane in the gas leaving the condenser.
3) A mixture of propane and butane is burned with air. Partial analysis of the stack gas produces the following dry-basis molar percentages: 0.0527% C3H8, 0.0527% C4H1O, 1.48% CO, and
7.12% CO2. The stack gas is at an absolute pressure of 780 mm Hg and the dewpoint of the gas is 46.5 Celsius. Calculate the molar composition of the fuel. A partially labeled diagram is
provided below.











