ลำดับที่...................
advertisement

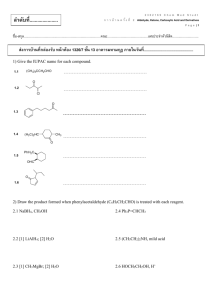
2302169 Chem Med Studt ก า ร บ้ า น ค รั ้ ง ที่ 1 S t e r e o c h e m i s t r y , A l c o h o l , E t h e r a n d E p o x i d e P a g e |1 ลาดับที่...................... ชื่อ-สกุล.....................................................................................คณะ.............................................เลขประจำตัวนิสติ ............................. ส่งการบ้านที่กล่องรับ หน้ าห้อง 1326/7 ชัน้ 13 อาคารมหามกุฏ ภายในวันที่................................................ 1) Label each pair of the following compounds as constitutional isomers, stereoisomers, or not isomers of each other (different compounds). O 1.1 O and 1.2 and O O CH 3 and 1.3 1.4 CH 3 and 2) On the basis of Cahn-Ingold-Prelog system, label each chiral center as R or S. SH 2.1 H 2.2 H CH3 CH3 CH 2 CN H H3 C 2.5 2.3 CH 3 D T Cl 2.6 Br CH2 I CH2 CH 3 H3 C H HO HOOC 2.7 H3 C HO H O 2.10 2 .17 2.13 2.14 O OH OH 2.8 Cl 2.12........... 2 .15 2.9 Cl 2.11........... 2 .16 O 2.11 NH 2 H CH 3 2.10........... OH 2.12 NH 2.4 2.9 .......... CH 3 O CH3 SH configuration (R or S) O O CH(CH 3 )2 O 2 .18 2.13........... O 2.14........... O O 2.15........... 2.16........... O 2.17........... 2.18........... Paclitaxel: a chemotherapy drug usually given to treat ovarian, breast and non-small cell lung cancer 2302169 Chem Med Studt ก า ร บ้ า น ค รั ้ ง ที่ 1 S t e r e o c h e m i s t r y , A l c o h o l , E t h e r a n d E p o x i d e P a g e |2 3) Threonine is a natural occurring amino acid that contains two stereogenic centers. Draw all possible stereoisomers and assign the R, S configuration to each isomer. H 2N H COOH H OH CH3 Threonine 4) How is each compound related to the simple sugar D-erythrose? Is it an enantiomer, diastereomer or identical compound? OHC H HO OH H CH 2OH OHC HO H CH 2OH H OH HOH2 C HO H 4.2 4.1 D-erythrose CHO OH H OHC HO H H OH CH 2OH OHC H HO 4.3 5) How are the compounds in each pair related to each other? (identical compound, enantiomers, diastereomers, constitutional isomers or different compound) and 5.1 CH 3 5.2 5.3 and CH 3 H3 C H HO CHO OH H OHC H HO and and 5.4 and 5.5 Cl Cl Cl 5.6 I Cl Br H and H Br I CH 3 H OH H OH CH 2OH 4.4 2302169 Chem Med Studt ก า ร บ้ า น ค รั ้ ง ที่ 1 S t e r e o c h e m i s t r y , A l c o h o l , E t h e r a n d E p o x i d e P a g e |3 5.7 H3 C H Br CH 3 Br H H3 C H Br and H Br CH 3 H 5.8 HO OH HO and OH H H H and 5.9 H 5.10 BrCH2 CH 3 CH2 OH CH 3 and 5.11 and HOH 2C H BrCH 2 H CH 3 CH3 H 5.12 HO CH 3 H CH 2 Br and H 3C Br CH 2OH H 6) The [α] of pure quinine, an antimalarial drug, is -165. Calculate the enantiomeric excess (ee) and % each enantiomer present in the solution with the following [α] values. 6.1) -50 N HO H 3CO 6.2) -83 N quinine 6.3) -120 2302169 Chem Med Studt ก า ร บ้ า น ค รั ้ ง ที่ 1 S t e r e o c h e m i s t r y , A l c o h o l , E t h e r a n d E p o x i d e P a g e |4 7) Give the IUPAC name for each alcohol. 7.1 (CH3 )2 CHCH2 CH2 CH 2OH 7.2 (CH3 )2 CHCH2 CH(CH 2CH3 )CH(OH)CH 2CH3 7.3 7.4 HO OH HO OH 7.5 OH 7.6 OH (H 3 C) 2HC OH 8) Rank each group in order of: 8.1) increasing boiling point:…………………………………………………… CH 3CH 2CH2OH a (CH 3) 2CHOH b CH 3CH 2OCH 3 c 8.2) increasing water solubility:………………………………………………… CH 3(CH 2)5OH a HO(CH2)6OH b CH 3(CH 2)4CH 3 c 9) Draw the organic product(s) formed when CH3CH2CH2OH is treated with each reagent. 9.1 H2SO4 9.2 NaH 9.3 HCl + ZnCl2 9.4 HBr 9.5 SOCl2, pyridine 9.6 PBr3 2302169 Chem Med Studt ก า ร บ้ า น ค รั ้ ง ที่ 1 S t e r e o c h e m i s t r y , A l c o h o l , E t h e r a n d E p o x i d e P a g e |5 9.7 TsCl, pyridine 9.8 [1] TsCl, pyridine; [2] NaSH 10) Identify compounds 10.1-10.6 in the following reactions 10.1 NaH TsCl, pyridine CH 3 I 10.2 10.3 CH 3 O- 10.4 10.5 CH 3 O- 10.6 HO H PBr3 10.7 How are compounds 10.2 and 10.4 related? 10.8 How are compounds 10.2 and 10.6 related? 11) Draw the products of each reaction. O 11.1 11.2 H3 C H 3C CH 3CH2 OH H H CH 3 H2 SO4 [1] CH 3CH2 ONa O [2] H2 O O HBr 11.3 O 11.4 [1] NaCN [2] H2 O 2302169 Chem Med Studt ก า ร บ้ า น ค รั ้ ง ที่ 1 S t e r e o c h e m i s t r y , A l c o h o l , E t h e r a n d E p o x i d e P a g e |6 12) Draw the products of each reaction, and indicate the stereochemistry when appropriate. OTs 12.1 KOC(CH 3 )3 OH 12.2 H HBr CH 3 O 12.3 CH 3CH2 H [1] NaCN H CH 2CH3 [2] H 2O OTs 12.4 (H C) C 3 3 KCN H PBr3 OH 12.5 H3 C OH 12.6 [2] CH3 CH 2O - H D 12.7 O [1] TsCl, pyridine HBr [1] NaOCH3 12.8 O [2] H 2O OH 12.9 CH 2 CH 3 CH3 12.10 H 3CH2 C C OCH 3 CH3 [1] NaH [2] CH3 CH2 I HI