Dose Modifications
advertisement
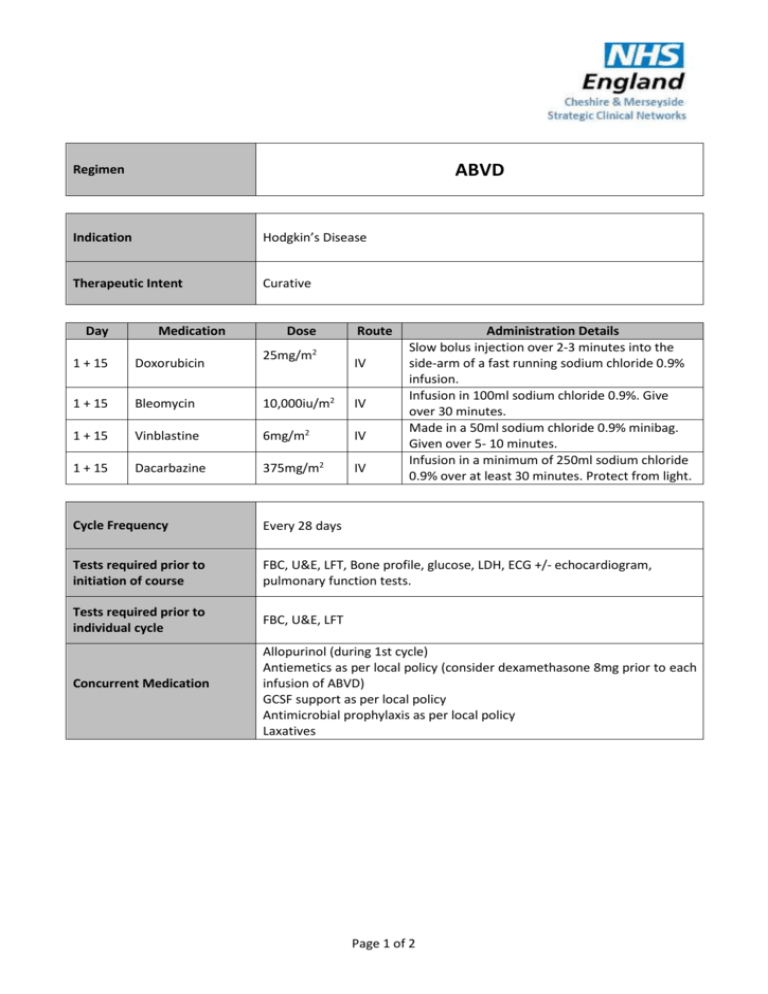
ABVD Regimen Indication Hodgkin’s Disease Therapeutic Intent Curative Day Medication Dose 25mg/m2 Route 1 + 15 Doxorubicin IV 1 + 15 Bleomycin 10,000iu/m2 IV 1 + 15 Vinblastine 6mg/m2 IV 1 + 15 Dacarbazine 375mg/m2 IV Administration Details Slow bolus injection over 2-3 minutes into the side-arm of a fast running sodium chloride 0.9% infusion. Infusion in 100ml sodium chloride 0.9%. Give over 30 minutes. Made in a 50ml sodium chloride 0.9% minibag. Given over 5- 10 minutes. Infusion in a minimum of 250ml sodium chloride 0.9% over at least 30 minutes. Protect from light. Cycle Frequency Every 28 days Tests required prior to initiation of course FBC, U&E, LFT, Bone profile, glucose, LDH, ECG +/- echocardiogram, pulmonary function tests. Tests required prior to individual cycle FBC, U&E, LFT Concurrent Medication Allopurinol (during 1st cycle) Antiemetics as per local policy (consider dexamethasone 8mg prior to each infusion of ABVD) GCSF support as per local policy Antimicrobial prophylaxis as per local policy Laxatives Page 1 of 2 Dose Modifications Hepatic Serum Bilirubin (micromol/L) Modification 1.7 – 2.5 x upper limit of normal 2.5 – 4 x upper limit of normal 50% doses of doxorubicin and vinblastine. 25% doses of doxorubicin and vinblastine. Renal Limited information – clinical decision Haematological No dose modifications for first cycle Neutrophils (x109/L) Recent studies have indicated that dose intensity can be maintained by administering ABVD irrespective of neutrophil count and without the use of GCSF. There are concerns that GCSF may enhance bleomycin induced lung toxicity. It is recommended that ABVD be delivered on schedule irrespective of neutrophil count and that clinicians show caution with respect to the use of GCSF. Platelets (x109/L) Modification >75 No dose modification <75 Delay all drugs for 1 week and repeat FBC Grade 2 motor weakness or Grade 3 sensory toxicity – give 50% vinblastine Higher grades of neurological toxicity – omit vinblastine Patients with any signs or symptoms of bleomycin pulmonary toxicity must have pulmonary function tests and a chest X-ray. Bleomycin should be discontinued if the diffusing capacity is <50% of the predicted value (or the chest X-ray shows evidence of pulmonary infiltrates or fibrosis) Check LV ejection fraction if any chest Xray during therapy shows cardiomegaly, or if signs or symptoms of congestive cardiac failure develop. If LVEF <50% - discontinue doxorubicin N.B. the maximum cumulative dose of doxorubicin is 450 – 500mg /m2 – account should be taken of other anthracyclines the patient may have received. In the presence of severe (≥grade 3) stomatitis or mucositis, delay therapy until ≤grade 1. In the presence of severe (≥grade 3) vinblastine-related ileus, delay treatment until recovery, and then give 75% vinblastine. If recurrent ≥grade 3 ileus develops, discontinue vinblastine Neurotoxicity Pulmonary Toxicity Cardiac dysfunction Gastro-intestinal toxicity Additional Information Irradiated blood products to be used References Adapted from NRCI RATHL trial protocol version 4.0 Author Pharmacy CNG Approved & Checked by Haematology CNG (Review Date = Sept 2017) Page 2 of 2