1308adv_abridged1
advertisement

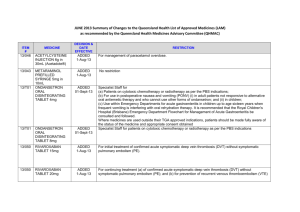
AUGUST 2013 Summary of Changes to the Queensland Health List of Approved Medicines (LAM) as recommended by the Queensland Health Medicines Advisory Committee (QHMAC) ITEM # MEDICINE DECISION & DATE EFFECTIVE RESTRICTION 13/071 IPILIMUMAB INJECTION 360mg (maximum amount) ADDED 01-Aug-13 Specialist Staff for use as per the Efficient Funding of Chemotherapy – Section 100 Arrangements Supplement of the PBS (Refer to www.pbs.gov.au) 13/070 OMEPRAZOLE MAGNESIUM TABLET 10mg ADDED 01-Oct-13 Specialist Staff and Country Medical Superintendents for oral administration in children under 1 year of age. (Tablets do not disperse evenly; not suitable for use in 6Fr NG tube - use omeprazole suspension) 13/069 PNEUMOCOCCAL CONJUGATE VACCINE (13VALENT) INJECTION, Pre-filled syringe 0.5mL (Prevenar 13®) ADDED 01-Oct-13 Adults at increased risk of invasive pneumococcal infection due to functional or anatomical asplenia. 13/083 RALTEGRAVIR Tablet 25mg, 100mg ADDED 01Sep-13 (to be reflected in 1 Oct LAM) Specialist Staff for use in accord with Highly Specialised Drugs Program indications 13/048 SODIUM CHLORIDE COMPOUND (Ringers lactate with glucose) Powder for reconstitution – sachet (4) plus bottle with paediatric tip (FLO kids Sinus Care Starter Kit®) ADDED 01-Oct-13 For initial supply post ENT surgery and sinus irrigation for children with chronic rhinosinusitis which may co-exist with cystic fibrosis and asthma. [Note: This item was discussed again at the September 2013 QHMAC meeting and further changes to its restriction were recommended. Refer to the September 2013 Summary of Changes, when available, for the revised LAM restriction]. ITEM # MEDICINE DECISION & DATE EFFECTIVE RESTRICTION VITAMIN, FAT SOLUBLE COMPOUND IN TOCOPHEROL PEG SUSPENSION chewable tablet (60), soft gel capsule (60), paediatric liquid 60mL (AquADEKs) AMISULPRIDE ORAL LIQUID 100 mg per mL, TABLET 100mg, 200mg, 400mg ADDED 01-Oct-13 Paediatricians, in consultation with RCH’s Paediatric Gastroenterology and Hepatology Service or Cystic Fibrosis Clinic, for infants and children with cholestasis (biliary atresia, alagilles, cystic fibrosis) and FSV deficiency resistant to standard oral FSV supplements. (Children should have documented deficiency of Vit K [abnormal Prothrombin time correctable with Vit K IMI], A, D and E on blood testing.) Where an item is not TGA approved, patients should be made fully aware of its status and appropriate consent obtained”. AMENDED 01-Oct-13 For use as per the PBS indications following failure of risperidone or olanzapine 13/076 APIXABAN Tablet 2.5mg For orthopaedic prophylaxis of venous thromboembolism in total hip and knee replacement 13/073B ARIPIPRAZOLE TABLET 5mg, 10mg, 15mg, 20mg, 30mg AMENDED 01-Sep-13 (to be reflected in 1 Oct LAM) AMENDED 01-Oct-13 13/073C ASENAPINE WAFER 5mg, 10mg AMENDED 01-Oct-13 For use as per the PBS indications following failure of risperidone or olanzapine 13/073D OLANZAPINE TABLET 2.5mg, 5mg, 7.5mg, 10mg; WAFER 5mg, 10mg, 20mg AMENDED 01-Oct-13 For use as per the PBS indications 13/051 13/073A For use as per the PBS indications following failure of risperidone or olanzapine Page 2 of 6 ITEM # MEDICINE DECISION & DATE EFFECTIVE RESTRICTION 13/070A OMEPRAZOLE MAGNESIUM TABLET 20mg AMENDED 01-Oct-13 Specialist Staff and Country Medical Superintendents for oral administration in children under 1 year of age. (Tablets do not disperse evenly; not suitable for use in 6Fr NG tube - use omeprazole suspension) 13/073E QUETIAPINE IMMEDIATE RELEASE TABLET 25mg, 100mg; MODIFIED RELEASE TABLET 50mg, 200mg, 300mg, 400mg RISPERIDONE TABLET and ORALLY DISINTEGRATING TABLET 0.5mg, 1mg, 2mg, 3mg, 4mg; ORAL LIQUID 1mg per mL, 100mL VITAMIN COMPOUND WITH MINERALS, CHEWABLE TABLET (Centrum Kids® Chewable Tablets) AMENDED 01-Oct-13 For use as per the PBS indications following failure of risperidone or olanzapine AMENDED 01-Oct-13 For use as per the PBS indications AMENDED 01-Oct-13 Patients unable to swallow tablets 13/073G ZIPRASIDONE CAPSULE 20mg, 40mg, 60mg, 80mg AMENDED 01-Oct-13 For use as per the PBS indications following failure of risperidone or olanzapine 13/028 CINACALCET TABLET 30mg, 60mg, 90mg 13/073F 13/063E DEFERRED Page 3 of 6 ITEM # 13/066 13/074 13/063A 13/063C 13/063B 13/063D MEDICINE DECISION & DATE EFFECTIVE LACTOBACILLUS ACIDOPHILUS & bifidobacterium bifidum CAPSULE 250mg SEVOFLURANE INHALATION ANAESTHETIC 250mL ALPROSTADIL INJECTION 5microgram in 1mL DEFERRED BISACODYL MAGNESIUM CITRATE (Pack containing 3 x 5mg bisacodyl tablets plus 1 x 10mg bisacodyl suppository and 1 x sachet magnesium citrate effervescent [equiv to 3g magnesium oxide (Go Kit Plus®) BISACODYL PAEDIATRIC SUPPOSITORIES 5mg (6) SODIUM HYALURONATE Intraocular Solution for Injection 10mg per mL, 1mL DELETED 01-Oct-13 RESTRICTION DEFERRED DELETED 01-Oct-13 DELETED 01-Oct-13 DELETED 01-Oct-13 Page 4 of 6 ITEM # MEDICINE DECISION & DATE EFFECTIVE 13/075 PALIVIZUMAB POWDER FOR INJECTION 50mg, 100mg NOT ADDED 13/067 RISEDRONATE SODIUM TABLET ENTERIC COATED 35mg NOT ADDED 13/049 SODIUM CHLORIDE COMPOUND (Sodium chloride, sodium bicarbonate, potassium chloride, calcium, magnesium chloride hexahydrate) Nasal Spray 15mL (FLO Baby Saline + Nasal Spray) GABAPENTIN TABLET 300mg NOT ADDED COUNCIL OF AUSTRALIAN THERAPEUTIC ADVISORY GROUPS (CATAG) UPDATE CYCLOPHOSPHAMIDE Tablet 50mg FOR INFORMATION HYDROXYETHYL STARCH Solutions for infusion NOTED 13/068 7. 13/072 13/065 RESTRICTION NOT AMENDED NOTED NOTED Page 5 of 6 ITEM # MEDICINE DECISION & DATE EFFECTIVE 13/019 MAGNESIUM SULFATE in water for Injection, prefilled syringe (PFS) 4g in 20mL (20%), and 10g in 50mL (20%) NOTED 12/T09 SOA – LOW MOLECULAR WEIGHT HEPARINS (LMWHs) NOTED RESTRICTION Page 6 of 6