1306adv_abridged
advertisement

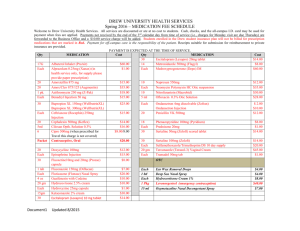
JUNE 2013 Summary of Changes to the Queensland Health List of Approved Medicines (LAM) as recommended by the Queensland Health Medicines Advisory Committee (QHMAC) ITEM # MEDICINE DECISION & DATE EFFECTIVE RESTRICTION 13/046 ACETYLCYSTEINE INJECTION 6g in 30mL (Acetadote®) ADDED 1-Aug-13 For management of paracetamol overdose. 13/043 METARAMINOL PREFILLED SYRINGE 5mg in 10mL ONDANSETRON ORAL DISINTEGRATING TABLET 4mg ADDED 1-Aug-13 No restriction 13/T01 ADDED 01-Sept-13 ADDED 01-Sept-13 Specialist Staff for (a) Patients on cytotoxic chemotherapy or radiotherapy as per the PBS indications; (b) For use in postoperative nausea and vomiting (PONV) (i) in adult patients not responsive to alternative oral antiemetic therapy and who cannot use other forms of ondansetron; and (ii) in children; (c) Use within Emergency Departments for acute gastroenteritis in children up to age sixteen years when frequent vomiting is interfering with oral rehydration therapy. It is recommended that the Royal Children's Hospital (Brisbane) Emergency Department Flowchart for Management of Acute Gastroenteritis be consulted and followed. Where medicines are used outside their TGA approved indications, patients should be made fully aware of the status of the medicine and appropriate consent obtained Specialist Staff for patients on cytotoxic chemotherapy or radiotherapy as per the PBS indications 13/T01 ONDANSETRON ORAL DISINTEGRATING TABLET 8mg 13/050 RIVAROXABAN TABLET 15mg ADDED 1-Aug-13 For initial treatment of confirmed acute symptomatic deep vein thrombosis (DVT) without symptomatic pulmonary embolism (PE). 13/050 RIVAROXABAN TABLET 20mg ADDED 1-Aug-13 For continuing treatment (a) of confirmed acute symptomatic deep vein thrombosis (DVT) without symptomatic pulmonary embolism (PE); and (b) for prevention of recurrent venous thromboembolism (VTE) ITEM # MEDICINE DECISION & DATE EFFECTIVE RESTRICTION 13/006 SUGAMMADEX SODIUM INJECTION 200mg in 2mL ADDED 01-Aug-13 For paediatric use by credentialed anaesthetists and Emergency Physicians for emergency reversal of rocuronium in children who cannot be intubated or cannot be oxygenated. [Only limited stock may be held outside pharmacy - on the difficult intubation trolley. All other use of sugammadex must be on an individual patient approval basis, with approval by the Director of Anaesthetics, the Director of Emergency Medicine or nominated representative, and in line with the SWAPNET guideline. (Available at http://qheps.health.qld.gov.au/patientflow/docs/swapnet_sugam_guide.pdf) Specialist Staff and Country Medical Superintendents for the treatment of cardiac glycoside intoxication resulting in life threatening cardiac arrhythmias; cardiac arrest; cardiac decompensation with hypotension; serum potassium >5.5mmol/L with cardiovascular instability or compromise. In the absence of these recognised indications of cardiac glycoside toxicity, digoxin immune Fab is not indicated based solely on a serum digoxin concentration. Specialist Staff for patients on cytotoxic chemotherapy or radiotherapy as per the PBS indications 13/018 A2 DIGOXIN IMMUNE Fab (Ovine) Powder for injection 40mg (DigiFab) AMENDED 01-Aug-13 13/T01 GRANISETRON INJECTION 3mg in 3mL AMENDED 01-Sept-13 13/012 A HUMAN C1 ESTERASE INHIBITOR Powder for injection 50unit per mL, 500unit AMENDED 01-Aug-13 Immunology physicians or Emergency Department physicians, after consultation with an immunology physician, for the treatment of patients with previously confirmed hereditary angioedema for: a) Acute attacks of angioedema affecting the upper respiratory tract or persistent abdominal attack resulting in significant intestinal obstruction; and b) Prophylaxis prior to significant dental and surgical procedures or for symptomatic attacks during pregnancy. (NOTE: Safety in pregnancy has not been established. Use only if indicated). 13/012 ICATIBANT Injection, single use pre-filled syringe 30mg in 3mL AMENDED 01-Aug-13 Immunology physicians or Emergency Department physicians, after consultation with an immunology physician, for the treatment of patients with previously confirmed hereditary angioedema for: a) Acute attacks of angioedema affecting the upper respiratory tract or persistent abdominal attack resulting in significant intestinal obstruction; and b) For outpatient use as per the PBS indications. 13/T01 ONDANSETRON INJECTION 4mg in 2mL; 8mg in 4mL AMENDED 01-Sept-13 Specialist Staff for (a) Patients on cytotoxic chemotherapy or radiotherapy as per the PBS indications; (b) Use for postoperative nausea and vomiting (PONV) Page 2 of 5 ITEM # MEDICINE DECISION & DATE EFFECTIVE RESTRICTION 13/T01 ONDANSETRON SYRUP 4mg in 5mL AMENDED 01-Sept-13 Specialist Staff for use in children who cannot use any other forms of ondansetron. Where medicines are used outside their TGA approved indications, patients should be made fully aware of the status of the medicine and appropriate consent obtained. 13/T01 ONDANSETRON TABLET 4mg AMENDED 01-Sept-13 13/T01 ONDANSETRON TABLET 8mg AMENDED 01-Sept-13 Specialist Staff for (a) Patients on cytotoxic chemotherapy or radiotherapy as per the PBS indications; (b) For use in postoperative nausea and vomiting (PONV) (i) in adult patients not responsive to alternative oral antiemetic therapy; and (ii) in children.Where medicines are used outside their TGA approved indications, patients should be made fully aware of the status of the medicine and appropriate consent obtained. Specialist Staff for patients on cytotoxic chemotherapy or radiotherapy as per the PBS indications. 13/028 CINACALCET TAB 30mg, 60mg, 90mg DEFERRED 13/048 SODIUM CHLORIDE COMPOUND (Ringers solution lactated with glucose) Powder for reconstitution – sachet (4) plus bottle with paediatric tip (FLO kids Sinus Care®) SODIUM CHLORIDE COMPOUND (sterile, isotonic), paediatric nasal spray,15mL VITAMIN, FAT SOLUBLE COMPOUND IN DEFERRED 13/049 13/051 DEFERRED DEFERRED Page 3 of 5 ITEM # 13/T03 MEDICINE TOCOPHEROL PEG SUSPENSION, chewable tablet, soft gel capsule, paediatric liquid 60mL ANIDULAFUNGIN Injection 100mg DECISION & DATE EFFECTIVE DELETED 01-Sept-13 13/T01 GRANISETRON INJECTION 1mg in 1mL DELETED 01-Sept-13 13/044 LEPIRUDIN INJECTION 50mg ONDANSETRON WAFER 4mg;8mg DELETED 01-Aug-13 DELETED 01-Sept-13 13/T01 13/T02 13/047 13/042 RESTRICTION SIMVASTATIN TABLET 80mg INDACATEROL CAPSULE, POWDER FOR INHALATION, 150microgram, 300microgram DELETED 01-Sept-13 NOT ADDED QUETIAPINE TABLET 200mg; 300mg NOT ADDED Page 4 of 5 ITEM # MEDICINE DECISION & DATE EFFECTIVE 13/T03 CASPOFUNGIN INJECTION 50mg; 70mg NOTED 13/045 FOR INFORMATION - CATAG SURVEY NOTED 13/025 TRICLOSAN Skin Cleanser, Soap 1% (125g) NOTED RESTRICTION Page 5 of 5