04-Beanium Activity
advertisement

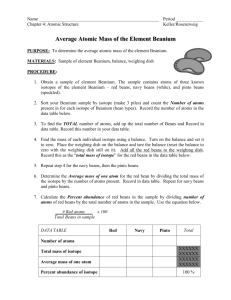
Beanium Activity Learning Target Introduction Materials Name/Number:_________________________________ Students will calculate the average atomic mass for an element. This activity is a simulation to determine the average atomic mass of an element. As atoms are too small to be seen, beans will be used to represent atoms of a new element. The new element, called beanium, is composed of three different isotopes. You will determine the percent abundance for each of the three isotopes and the mass of one atom of each isotope; this information will then be used to determine the average atomic mass of the element beanium. sample of beanium balance Procedure 1. Obtain a beanium sample that has been well mixed and contains the three isotopes. 2. Separate your sample into the three isotopes. Count the number of atoms (beans) for each isotope. Record the numbers in the data table. 3. Calculate the total number of all the isotopes (beans) in your sample. Record this above your data table. 4. Calculate the percent abundance for each of the three isotopes by using the formula: % = (part/whole) x 100. Note: For significant figures, keep the percent to the hundredths. 5. Although you need the mass of one of each of the isotopes, one atom is too small (and in this lab’s case too variable) to get an accurate mass. Instead, find the total mass of all of the atoms for isotope 1 and record the TOTAL mass in your data table. 6. Measure and record the total mass of the other two isotopes and record in the data table. 7. Return your beans to the container and clean up your lab station. Be sure to wash your hands. Check out with your instructor. 8. Calculate the mass of one atom of each isotope using the total mass and the number of beans of that particular isotope. Record this number in the data table. Base your number of significant figures off the total mass of the sample, not the number of beans. Data Total number of isotopes (beans):__________ Isotope 1 Isotope 2 Isotope 3 Description of Isotope Number of atoms in sample Percent abundance Total mass Mass of one atom Calculations Be sure to show all of your work and include units. 1. Determine the average atomic mass of one atom of beanium using the following formula. Do NOT use the total mass for each isotope as the mass – use the mass of one atom. average atomic mass = (percent / 100) x mass + (percent / 100) x mass + (percent / 100) x mass Questions Answer each question thoroughly in complete sentences. 1. What was different about each “isotope” of beanium? What was the same? Do not discuss the color or shape of the beans for this question. Use the numbers from the data table and information in the introduction. 2. Why was it important that the beanium was well mixed before the samples were taken? 3. Explain why the weighted average atomic mass of the element ‘Beanium’ does not equal the mass of any one piece in the sample. 4. Gallium consists of two naturally occurring isotopes, gallium-69 [68.93 amu] and gallium-71 [70.95 amu]. a. How many protons and neutrons are in the nucleus of each isotope? This question does not have to be in a complete sentence. b. Look up the atomic mass of gallium [Ga] on a periodic table. How does it compare to the masses of the isotopes of gallium? Be sure to include the average atomic mass of gallium from the periodic table in your answer. c. Does it look like the element is made up of exactly 50% of each isotope? How do you know?