Lab 4: Determination of an Equilibrium Constant using Spectroscopy
advertisement

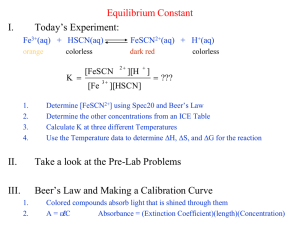
Lab 4: Determination of an Equilibrium Constant Objectives: To determine the concentration of iron (III) thiocyanate ions, FeSCN2+, in various iron (III) nitrate, Fe(NO3)3, and potassium thiocyanate, KSCN solutions. The results of these measurements will determine the equilibrium constant for the formation of FeSCN2+. Introduction: Chemical reactions occur to reach a state of chemical equilibrium. The equilibrium state can be characterized by specifying its equilibrium constant. In this experiment you will determine the value of the equilibrium constant for the reaction between the iron (III) ion, Fe3+, and thiocynate ion, SCN-. Fe3+(aq) + SCN-(aq) FeSCN2+(aq) (1) where 𝐾𝑒𝑞 = [𝐹𝑒𝑆𝐶𝑁2+ ] [𝐹𝑒 3+ ][𝑆𝐶𝑁 − ] (2) To find the value of Keq, it is necessary to determine the concentration of each of the three species in solution at equilibrium. This can be done using UV-visible spectroscopy. Part A - Determination the Calibration Curve of FeSCN2+ by Spectrometry When a chemical reaction reaches chemical equilibrium, the rates of the forward and the reverse reactions are equal. The concentrations of all the species become constant. In this experiment, we take advantage of the fact that FeSCN2+ is a coloured compound and that the concentration of this compound can be determined by measuring its absorbance using spectrophotometric methods. This method requires a calibration curve using samples of known concentration. Beer's law relates absorbance, A, the light absorbed on passing through a length of solution, , of a concentration, c, of the absorbing solute. According to Beer's law, the amount of light absorbed by a coloured species in solution at a specific wavelength is directly proportional to the concentration of the coloured species. A=ε··c (3) The absorbance is measured for a series of standard solutions with known concentrations. If a solute obeys Beer's law, according to equation (3), a graph of the "Absorbance versus Concentration" will yield a straight line of slope equal ε . This is known as a calibration curve for the solute. From the calibration curve, the absorbance of the solute with an unknown concentration can be determined. In usual practice of this technique, a blank is used to cancel out absorption by any other solutes, so that the measured absorbance is directly proportional to the concentration of the solute of interest. This is routinely done by people working in the fields of medicine, forensic science, and chemistry. Document1 4-1 In this lab, the solute of interest is FeSCN2+. A series of standard FeSCN2+ solutions will be prepared from solutions of varying concentrations of SCN- and constant, high concentrations of Fe3+ and H+. The high concentration of the Fe3+ ion, relative to that of the SCN- ion, drives equilibrium (1) to the right; essentially to completion. Solutions of Fe3+ are weakly coloured and the SCN- ion is colourless. The primary absorber in the mixture will be the FeSCN2+. FeSCN2+ is known to obey Beer's law over a wide range of concentrations with maximum absorption at a wavelength of 447 nm. Thus, this wavelength will be used to make measurements of the FeSCN2+ concentration in equilibrium mixtures. The high [H+] will ensure that the iron (III) ion will not form the insoluble compound, iron (III) hydroxide. The high [Fe3+] will ensure that all SCNreacts to form FeSCN2+. The FeSCN2+ complex forms slowly in about 1 minute and then it decomposes slowly due to its reaction with light. For best results, the absorbance value should be read between 2 and 4 minutes after preparation and all samples read after the same time interval. Part B - Determination of an Equilibrium Constant A second series of solutions will be prepared for the determination of the equilibrium constant. These solutions will have a fixed high concentration of H+, a constant low concentration of Fe3+ and varying low concentration of SCN-. The absorbance readings will enable the equilibrium concentration of FeSCN2+ to be determined directly. The Fe3+ ion is obtained from a solution of Fe(NO3)3 which total dissociates to give Fe(NO3)3(aq) Fe3+(aq) + 3 NO3-(aq). Therefore the initial [Fe3+]o = [Fe(NO3)3]o. Similar, the SCN- ion is obtained from a solution of KSCN which total dissociates to give KSCN(aq) K+(aq) + SCN-(aq). Therefore the initial [SCN-]o = [KSCN]o. The equilibrium concentrations of Fe3+ and SCN- can be calculated from their initial concentrations and the equilibrium concentration of FeSCN2+. Procedure: Part A - Determination the Calibration Curve of FeSCN2+ by Spectrometry Note: For Part A of the experiment, we will be using the 0.150 M Fe(NO3) 3 and 5.00x10-4 M KSCN solutions. Assume that all of the SCN- is present as the complex ion, FeSCN2+ due to the high concentration of Fe3+. Work in pairs. Deliver all volumes of Fe3+ solutions with a 10.0 mL bottle top dispenser and SCNsolutions using a burette. Add the solutions into a 25.00 mL volumetric flask and dilute to the mark with 0.10 M nitric acid. All cuvettes should be wiped clean and dry on the outside with a tissue. All solutions should be free of bubbles. Always position the cuvette with its reference mark facing the reference mark on the spectrophotometer. Document1 4-2 1. Turn on the spectrophotometer, set Mode to Absorbance, and set the wavelength to 447 nm. 2. Prepare solutions according to Table 1 using 25.00 mL volumetric flasks. Dilute to the mark with 0.10 M HNO3 and mix well. Solutions # mL of 0.150 M Fe(NO3)3 in 0.10 M HNO3 use a bottle-top dispenser mL of 5.00x10-4 M KSCN in 0.10 M HNO3 use a burette 1 (Blank) 10.00 0 2 3 4 5 6 10.00 10.00 10.00 10.00 10.00 2.00 3.00 5.00 7.00 9.00 Table 1: Volume of reagents used to make solutions. 3. Obtain a clean cuvette and rinse (2x with small portions) with Solution #1, the blank solution, and fill the cuvette ¾ full. Wipe the outside of the cuvette and avoid touching the bottom half of the cuvette. Insert the cuvette into the sample compartment with its orientation mark aligned with the mark in the spectrophotometer and set the absorbance to read 0. This is the absorbance of unreacted Fe3+ in the solution. All the standards have a large excess of Fe3+, which absorbs light in this wavelength region. 4. Empty the cuvette and rinse (2x with. small portions) with Solution #2 and fill the cuvette ¾ full. Wipe the outside of the cuvette and avoid touching the bottom half of the cuvette. 5. Insert the cuvette filled Solution #2 and read the absorbance of the solution. 6. Repeat Step 4 and 5 to prepare and measure the absorbance for Solutions #3 to #6 in order of increasing SCN- concentration and read the absorbance of each sample once the solution is prepared. Remember to rinse the cuvette with each sample. 7. Check that the absorbance values are in direct proportion with one another so the graph will be linear. Prepare alternate solutions to any which fall outside the linear region and measure the absorbance. 8. Calculate [FeSCN2+] for each solution and enter the concentrations on the datasheet. Show a sample calculation for Solution #3 9. Plot a calibration graph of net absorbance versus [FeSCN2+]. Include the (0, 0) point. Clearly label the graph. Obtain the equation of the straight line for the calibration graph written in terms of the variables used in this experiment. Attach the graph to the report sheet. Document1 4-3 Part B - Determination of an Equilibrium Constant Note: For Part B of the experiment, we will be using the 1.50 x 10-3 M Fe(NO3) 3 and 3.00x10-3 M KSCN solutions. Work in pairs. Deliver all volumes of Fe3+ solutions with a 10.0 mL bottle-top dispenser and SCN solutions using a burette. Add the solutions into a 25.00 mL volumetric flask and dilute to the mark with 0.10 M nitric acid. All cuvettes should be wiped clean and dry on the outside with a tissue. All solutions should be free of bubbles. Always position the cuvette with its reference mark facing the reference mark on the spectrophotometer. 1. Prepare the solutions shown in Table 2 and measure the absorbance of each using Solution #7 as the new blank (set absorbance to 0 with Solution #7). As in Part A, measure the absorbance of the solution once it has been prepared. Solutions # mL of 1.50x10-3 M Fe(NO3)3 in 0.10 M HNO3 use a bottle-top dispenser mL of 3.00x10-3 M SCN- in 0.10 M HNO3 use a burette 7 (Blank) 10.00 0 8 9 10 11 12 10.00 10.00 10.00 10.00 10.00 2.00 3.00 5.00 7.00 8.00 Table 2: Volume of reagents used to make solutions. 2. Prepare alternate solutions to any which have absorbencies outside the linear region of Beer’s Law. 3. Determine the [FeSCN2+] at equilibrium from the calibration graph and measured absorbance. Enter the concentration values on the datasheet. 4. Calculate the initial concentrations of Fe3+ and SCN- for Solutions #8 to #12. Enter the concentration values on the datasheet. 5. Calculate the equilibrium concentrations of Fe3+ and SCN- for Solutions #8 to #12. Enter the concentration values on the datasheet. 6. Calculate the equilibrium constant, Keq, for Solutions #8 to #12. Enter the values on the datasheet. Show a sample calculation of Keq for Solution #10. 7. Calculate the average Keq and the standard deviation in Keq. Document1 4-4 Lab 4: Determination of an Equilibrium Constant Name:__________________________ Date: ________ Partner: _____________ Purpose: Data and Results Part A –Determination the Calibration Curve of FeSCN2+ by Spectrometry Solutions # [FeSCN2+] (M) 1 0 (blank) Absorbance 2 3 4 5 6 For solution 3# show the calculation of [FeSCN2+]. Document1 4-5 Part B - Determination of an Equilibrium Constant Solutions # [FeSCN2+] (M) 7 0 (blank) Absorbance 8 9 10 11 12 Solution # Initial [Fe3+] (M) Initial [SCN-] (M) Equilibrium [FeSCN2+](M) Equilibrium [Fe3+] (M) Equilibrium [SCN-] (M) Keq 8 9 10 11 12 Average Keq = ______________________ Standard Deviation of Keq = ______________________ Document1 4-6 Show a sample calculation of Keq for Solution #10. Include all the steps. Conclusions: Document1 4-7