Colorimetric Analysis & Equilibrium Help Notes - AP Chem
advertisement

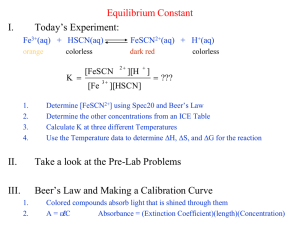
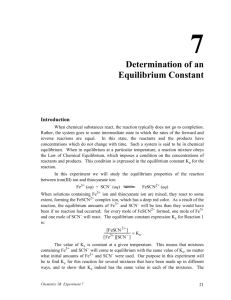
Colorimetric Analysis & Determination of the Equilibrium for a Chemical reaction Help Notes AP Chemistry PART II How to determine Concentration of FeSCN2+ at equilibrium. •In this step you are determining the concentration of the product at equilibrium using the absorbance reading you measured in the data table. •Use the calibration curve you generated in Part I to determine the concentration of your samples. Plug in the y value for absorbance to find the x value for concentration. PART II How to determine Concentration of FeSCN2+ at equilibrium. •Here is one set of sample data generated in class. Your absorbance values should be DETERMINING y WHEN GIVEN x Steps for TI Graphing Calculators The directions below are form your Arrhenius made easy notes. You will use the same steps to find x except instead of looking for Kelvin temperature your x will represent the concentration of your sample at equilibrium. How to determine the initial concentrations of diluted stock standard. In this step you are determining the INITIAL concentrations of reagents added to the reaction. You will use the calibration curve you generated in Part I to determine the equilibrium concentrations AFTER the reaction has completed. Tube # 0.002 M Fe(NO3)3 (mL) 0.002 M SCN(mL) 1 3.00 0.00 7.00 2 3.00 2.00 5.00 3 3.00 3.00 4.00 4 3.00 4.00 3.00 5 3.00 5.00 2.00 H2 O (mL) Initial [Fe3+] Initial [SCN-] ? ? ? ? ? ? ? ? ? ? How to determine the initial concentrations concentration of diluted stock standard. FIRST: Determine the total volume of the new, diluted concentration. To do this, add up all the amounts of standard added. Tube # 0.002 M Fe(NO3)3 (mL) 0.002 M SCN(mL) 1 3.00 0.00 7.00 2 3.00 2.00 5.00 3 3.00 3.00 4.00 4 3.00 4.00 3.00 5 3.00 5.00 2.00 H2 O (mL) Initial [Fe3+] Initial [SCN-] ? ? ? ? ? ? ? ? ? ? Note that for every sample the total volume is 10 ml = 0.01 L. How to determine the initial concentrations concentration of diluted stock standard. Second: Calculate the molar concentration of Fe(NO3)3 in each tube. Tube # 0.002 M Fe(NO3)3 (mL) 0.002 M SCN(mL) 1 3.00 0.00 2 3.00 3 3.00 4 3.00 5 3.00 H2 O (mL) 7.00 Initial [Fe3+] Initial [SCN-] ? ? 2.00 5.00 ? ? 3.00 4.00 ? concentration ? Note that this will be the 4.00 ? samples ? same3.00for ALL because you add 5.00 2.00 the same amount ? ? with the same final volume each time. How to determine the initial concentrations concentration of diluted stock standard. Finally: Calculate the molar concentration of SCN- in each tube at each concentration. Tube # 0.002 M Fe(NO3)3 (mL) 0.002 M SCN(mL) 1 3.00 0.00 2 3 4 5 H2 O (mL) 7.00 Initial [Fe3+] 6 x 10-4 Initial [SCN-] ? 6 x 10 3.00 2.00 5.00 For sample #1 it is 0.00 ? 6add x 10 any 3.00 3.00 4.00 because you didn’t ? 6 x 10 SCN-. 3.00 4.00 3.00 ? 6 x 10 3.00 5.00 2.00 ? -4 -4 -4 -4 How to determine the initial concentrations concentration of diluted stock standard. Finally: Calculate the molar concentration of SCN- in each tube at each concentration. Tube # 0.002 M Fe(NO3)3 (mL) 0.002 M SCN(mL) 1 3.00 0.00 2 3.00 2.00 3 3.00 3.00 4 3.00 4.00 5 3.00 5.00 Initial [Fe3+] Initial [SCN-] 7.00 6 x 10-4 0 5.00 6 x 10-4 H2 O (mL) For sample ?#2-5 find the 6 x 10 of stock solution and 4.00 amount ? calculate for? a new final 6 x 10 3.00 volume 6 x 10 of 10 mL (0.01 L) 2.00 ? -4 -4 -4 How to determine the initial concentrations concentration of diluted stock standard. Finally: Calculate the molar concentration of SCN- in each tube at each concentration. Tube # 0.002 M Fe(NO3)3 (mL) 0.002 M SCN(mL) 1 3.00 0.00 2 3.00 3 Initial [Fe3+] Initial [SCN-] 7.00 6 x 10-4 0 2.00 5.00 6 x 10-4 4 x 10-4 3.00 3.00 4.00 6 x 10-4 4 3.00 4.00 3.00 6 x 10-4 5 3.00 5.00 2.00 6 x 10-4 ? ? ? H2 O (mL) You will have to calculate the concentration for each sample since the amounts vary. However, final volume will remain 10 mL for each sample. PART II: Pre-Lab Questions (a) Write the balanced net ionic equation for the reaction taking place: (see pg 6) Fe3+ (aq) + SCN- (aq) FeSCN2+ (aq) PART II: Pre-Lab Questions (b) Write the equilibrium expression for the reaction taking place. Fe3+ (aq) + SCN- (aq) FeSCN2+ (aq) Remember, [products]coeff./ [reactants]coeff. PART II: Post Lab Questions 3. Use a RICE table along with the initial concentrations of the reactants you calculated in Part II and equilibrium concentrations to determine the value of Keq for each experimental trial of this experiment. PART II: Post Lab Questions RICE tables are a way of organizing your data to quickly solve equilibrium problems. R: Write the balanced net ionic equation. I: List the initial concentrations for each species (product and reactants) C: On this row you will figure out what the change in concentration for the reaction is. E: Here you will list the equilibrium concentrations for each species. PART II: Post Lab Questions For our Lab:. R: Write the balanced net ionic equation. I: C: E: PART II: Post Lab Questions For our Lab:. R: Fe3+ (aq) + I: C: E: SCN- (aq) FeSCN2+ (aq) PART II: Post Lab Questions For our Lab:. R: Fe3+ (aq) + SCN- (aq) FeSCN2+ (aq) I: List the initial concentrations. These are the values you calculated in table 2 on page 7. C: E: PART II: Post Lab Questions For our Lab Test tube 2: R: Fe3+ (aq) + SCNI: C: E: 6x10-4M (aq) 4x10-4 M FeSCN2+ (aq) 0 (initially there is no product!) PART II: Post Lab Questions For our Lab Test tube 2: R: Fe3+ (aq) + SCNI: 6x10-4M (aq) 4x10-4 M FeSCN2+ (aq) 0 (initially there is no product!) C: E: the equilibrium concentrations are the concentrations you measured in table 1, page 7. You calculated these concentrations from absorbance and your calibration curve. PART II: Post Lab Questions For our Lab Test tube 2: R: Fe3+ (aq) + SCNI: 6x10-4M (aq) 4x10-4 M FeSCN2+ (aq) 0 (initially there is no product!) C: E: 4.8x10-3-4 M PART II: Post Lab Questions For our Lab Test tube 2: R: Fe3+ (aq) + SCNI: 6x10-4M (aq) 4x10-4 M FeSCN2+ (aq) 0 (initially there is no product!) C: E: 4.8x10-3-4 M Do you remember why we are doing the RICE table? Our goal is to find Keq, the equilibrium constant. To do this we need to know the concentrations of ALL species at equilibrium. PART II: Post Lab Questions For our Lab Test tube 2: R: Fe3+ (aq) + SCNI: 6x10-4M (aq) 4x10-4 M FeSCN2+ (aq) 0 (initially there is no product!) C: E: 4.8x10-3-4 M We are missing the equilibrium concentrations of the reactants. BUT, we know the change in concentration and the ratios of those changes so we can calculate the final concentrations of our reactants. PART II: Post Lab Questions For our Lab Test tube 2: R: Fe3+ (aq) + SCNI: 6x10-4M (aq) 4x10-4 M FeSCN2+ (aq) 0 (initially there is no product!) C: That’s where “C” comes in. This represents the change in concentration as equilibrium is reached. E: 4.8x10-3-4 M PART II: Post Lab Questions For our Lab Test tube 2: R: Fe3+ (aq) + SCNI: 6x10-4M (aq) 4x10-4 M FeSCN2+ (aq) 0 (initially there is no product!) C: Use the coefficients to determine the mole ratio changes during the reactions. Our reaction is 1:1:1 so this problem is relatively simple. E: 4.8x10-3-4 M PART II: Post Lab Questions For our Lab Test tube 2: R: Fe3+ (aq) + SCNI: 6x10-4M (aq) 4x10-4 M FeSCN2+ (aq) 0 (initially there is no product!) C: Notice that if we produce one mole of Product then one mole of each reactant is consumed. E: 4.8x10-3-4 M PART II: Post Lab Questions For our Lab Test tube 2: R: Fe3+ (aq) + SCNI: 6x10-4M (aq) 4x10-4 M FeSCN2+ (aq) 0 (initially there is no product!) C: Let “x” represent this amount. E: 4.8x10-3-4 M PART II: Post Lab Questions For our Lab Test tube 2: R: Fe3+ (aq) + SCNI: 6x10-4M (aq) 4x10-4 M FeSCN2+ (aq) 0 (initially there is no product!) C: -x -x +x One mole of each reactant was consumed (-x) to produce one mole of product (+x) E: 4.8x10-3-4 M PART II: Post Lab Questions For our Lab Test tube 2: R: Fe3+ (aq) + SCNI: C: E: 6x10-4M -x (aq) 4x10-4 M -x FeSCN2+ (aq) 0 (initially there is no product!) +x 4.8x10-3-4 M Now determine the equilibrium concentrations after the “C” values are subtracted or added. Evaluating the product first we see the x = 4.8x10-3 PART II: Post Lab Questions For our Lab Test tube 2: R: Fe3+ (aq) + SCNI: C: E: 6x10-4M -x (aq) 4x10-4 M -x 6x10-4M - 3.8x10-4 4x10-4 - 3.8x10-4 2.2x10-4 2.5x10-5 FeSCN2+ (aq) 0 (initially there is no product!) +x 3.8x10-4 M Now we can subtract “X” from our starting concentrations of reactants PART II: Post Lab Questions For our Lab Test tube 2: R: Fe3+ (aq) + SCNI: C: E: 6x10-4M -x (aq) 4x10-4 M -x 6x10-4M - 3.8x10-4 4x10-4 - 3.8x10-4 2.2x10-4 2.5x10-5 FeSCN2+ (aq) 0 (initially there is no product!) +x 3.8x10-4 M Do you remember our goal? Find Keq . Now all we need to do is plug our equilb. Concs. Into the equlibrium expression! PART II: Post Lab Questions For our Lab Test tube 2: R: Fe3+ (aq) + SCNI: C: E: 6x10-4M -x (aq) 4x10-4 M -x 6x10-4M - 3.8x10-4 4x10-4 - 3.8x10-4 2.2x10-4 2.5x10-5 [FeSCN2+] Keq = [Fe3+][SCN-] = plug and chug! FeSCN2+ (aq) 0 (initially there is no product!) +x 3.8x10-4 M