EXPERIMENT 5 – - Seattle Central College
advertisement

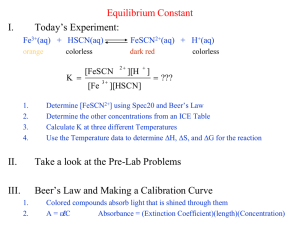
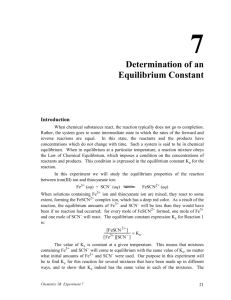
Experiment #4 Measurement of an Equilibrium Constant Adapted by Tom Schultz from Laboratory Experiments in Inorgrganic Chemistry by Seattle U. Inorganic Chemistry Faculty SUMMARY In this experiment, you determine the equilibrium constant of a reaction by using spectrophotometry to measure the equilibrium concentrations of all equilibrium species. LEARNING OBJECTIVES 1. 2. 3. 4. 5. WRITTEN WORK Determine molar absorptivity () for a specified substance. Use experimental data to calculate concentrations of specified reacting substances Calculate an equilibrium constant from experimental data. Properly use a volumetric pipette in making dilutions. Attain greater familiarity with spectroscopy. Pre-Lab Assignment (due at start of lab): Please answer the planning questions below and complete the rest of the pre-laboratory assignment, as described in the course information packet (i.e., with headings, purpose, and data,). Planning Questions: 1. Calculate the concentrations of the solutions you will prepare. 2. On page 5, step 2, you are instructed to take blank measurements before reading every sample. Why are you asked to do this and why are the blanks listed appropriate? In addition, please prepare data tables in advance (follow the examples provided) for entering the data you will measure in lab. Note: each FeSCN2+ mixture must be measured within two minutes of mixing, so you will not have time to make up the data tables as you go. Post-Lab Assignment Normal procedure as stated on the web site. SAFETY Potential hazards Observe good lab practices; safety glasses must be worn at all times. Waste disposal Your reaction mixture should be put in the labeled waste container on the side shelf or in the hood. -1- INTRODUCTION Your main goal in this experiment is to determine the equilibrium constant, K, for the following reaction: Eq. 1 Fe3+(aq) + SCN-(aq) FeSCN2+(aq) To accomplish this goal, you will find the equilibrium concentrations of Fe3+, SCN-, and FeSCN2+ in four different solutions and use them to calculate the value of K for each solution. Recall that K is given by the following expression: Eq. 2 To find the equilibrium concentrations in the expression above, you will use a spectrophotometer to measure the absorbance of FeSCN2+ in solution. Then, you will convert that absorbance to a concentration value using Beer’s Law. Finally, using the stoichiometric relationships between the chemical species at equilibrium, you will determine the concentrations of Fe3+ and SCN- and solve for K. (These calculations are described in more detail below.) A. Determining the molar absorptivity, ε, of FeSCN2+ In order to relate the absorbance of a FeSCN2+ solution to concentration values, you will need to determine the molar absorptivity, ε, of FeSCN2+. Because you must be able to convert absorbance readings into concentrations, you will start this lab by determining the value of ε for FeSCN2+. For solutions of known concentration, Beer’s Law can be used to solve for ε as long as the path length is known. (In this lab, assume the path length in the cuvette is 1.00 cm.) Eq. 3 Beer’s Law In the expression above, A is absorbance is the molar absorptivity (M-1cm-1), c is the concentration (M) of the absorbing species, and l is the path length (cm). Preparing a solution of known FeSCN2+ concentration To prepare a solution with a known concentration of FeSCN2+, you will use an equilibrium “trick” to create a solution with a specific concentration of FeSCN2+(aq). In a solution with a high initial concentration of Fe3+ and a very low initial concentration of SCN-, the equilibrium will shift almost completely to the right, with SCN- acting as a limiting reagent. The high amount of Fe3+ shifts the equilibrium to the product side, but -2- the low amount of SCN- limits the amount to which it can shift. Because the amount of FeSCN2+ produced is stoichiometrically equal to the amount of SCN- that reacts, [FeSCN2+]eq = [SCN-]eq. To see how this technique works, consider the reaction table for this equilibrium: Fe3+(aq) + Initial Change Equilibrium 0.20 M Fe3+ -x 0.20 M – x SCN-(aq) 0.0001 M SCN-x 0.0001 – x FeSCN2+(aq) 0 x x The large amount of Fe3+ shifts the equilibrium to the right, so that virtually all of the SCN- reacts. (Of course, a tiny amount of SCN- remains, but it is smaller than our degree of experimental precision). Thus, 0.0001 – x, the x can be ignored making [FeSCN2+]eq = x = 0.0001 M. Please note that this technique only works in this part of the lab due to the high concentration of Fe3+, as a way to create a solution of FeSCN2+ of known concentration. This allows the value of the molar absorptivity, ε to be calculated. The concentration of FeSCN2+ in the four other test solutions will vary but can be easily calculated from Beer’s law below. Determining ε from the solution of known FeSCN2+ concentration. Once c, and A are known, it is easy to solve for ε using Beer’s Law: The value of ε makes it possible to relate the absorbance (A) of an FeSCN2+ solution to the corresponding concentration, assuming that ε remains constant over a range of concentrations. PLEASE NOTE: Because the iron (III) ion, Fe3+ absorbs some light at the same wavelength as FeSCN2+, you will have to take special precautions to subtract the Fe3+ absorbance from your overall FeSCN2+ absorbance. The easiest way to do this is to prepare a blank containing ferric nitrate and use it to set the transmittance to 100% , this process called blanking will eliminate the Fe3+ absorbance from interfering with the measurement of the absorbance of the produce. This process is called “blanking”. Important: If this subtraction is to work, the blank must have the same concentration of Fe3+ as the solution you are measuring. You will need to run such blanks for EACH and EVERY solution you test with the spectrophotometer. Details on this process are in the procedure. B. Finding the equilibrium constant of four different solutions Fe3+ and SCN- are mixed together in different proportions in four different test tubes. After the contents of each test tube come to equilibrium (which happens almost instantaneously), you will measure the FeSCN2+ concentration of each and use it to -3- calculate the concentrations of the other species, as well as the equilibrium constant for each system. (All the K’s should be the same, since each test tube is at the same temperature.) Preparing the initial solutions The initial concentration of all solutions is outlined in the experimental procedure section of this document. Prior to coming to class these calculations need to be made. Fe3+ Solution # 1 2 3 4 5 [Fe3+]i 0.04 M 0.02 M 0.001 M 0.0005 M 0.00025 M [SCN-]i stock solution 0.0002 M Creating the equilibrium mixtures To create each equilibrium mixture, you will mix 5 ml of one of the Fe3+ solutions with 5 ml of the SCN- solution in a test tube (see the procedure for exact details). Please note that this mixing process will change the initial concentrations of each reactant. For example, if you mix 5 ml of the 0.04 M Fe3+ solution with 5 ml of the 0.0002 M SCNsolution, each solution will dilute the other to a total volume of 10 ml. Thus, each reactant will be at a smaller concentration, even before they start to react with each other to reach equilibrium. Recall that you can calculate the new concentration of a solution, after dilution, by using the following equation: C1V1 = C2V2 Eq. 4 Determining the equilibrium concentration of FeSCN2+ The spectrophotometer measures the absorbance due to FeSCN2+ in each test tube, while Beer’s Law can be used to convert that absorbance into [FeSCN2+] concentration. Determining the equilibrium concentration of SCN- and Fe3+ If the initial amounts of Fe3+ and SCN- are known, the reaction stoichiometry can be used to calculate their equilibrium values. An example of such a calculation is shown in the reaction table below: Fe3+(aq) + Initial Change Equilibrium 0.01 M -x 0.01 – x SCN-(aq) 0.0001 M -x 0.0001 – x FeSCN2+(aq) 0 x x The value of x in the FeSCN2+ column is determined directly by measuring the absorbance with the spectrophotometer. Once it is known, it can be used to calculate the equilibrium concentrations of Fe3+ and SCN-. -4- EXPERIMENTAL PROCEDURE Equipment and Chemicals Glassware for making dilutions (The following will be available: volumetric pipettes - 1.00 mL, 5.00 mL, 10.00 mL, and 25.00 mL; you will also have a 25.00 mL and 50.0 mL volumetric flasks.) 6 test tubes test tube rack, labels spectrophotometer 1 spectrophotometer cuvette 70 mL 0.0002M KSCN 30 mL 0.20M Fe(NO3)3 (stock solution) 5 small flasks or beakers Preparation of KSCN Subsamples (See website for directions on Spec-20 operation) 1. 2. Use a pipette to measure 5.0mL of 0.0002M KSCN solution into 5 test tubes. Label the test tubes A through E. Determination of molar absorptivity () 1. Prepare a 0.1M Fe3+ solution by diluting 5.0mL of 0.2M Fe(NO3)3 with 5.0mL H2O in a small beaker of flask. 2. Set the wave length on the spectrophotometer to 446nm. 3. Set transmittance to zero, with noting in the sample holder and the transmittance to 100% with a 0.1MFe3+ solution in the sample holder. This process is called blanking. 4. Add 5.0 mL of 0.2M Fe(NO3)3 to test tube A (which already contains 5.0 mL of KSCN from step 1 above). 5. Swirl the test tube to mix and immediately transfer some to a cuvette. 6. Place the cuvette in the sample holder, switch to absorbance, and record the absorbance in your notebook. Preparation of Dilute Fe(NO3)3 Solutions 1. Label 5 small flasks or beakers 1 through 5. 2. Use volumetric pipettes and flasks to prepare 50.0mL of 0.04M Fe(NO3)3 using your 0.2M stock solution. Before diluting, add 2 % by volume of 0.05M HNO3. Mix well. This is solution 1. You will not add additional HNO3 to subsequent samples. 3. Use volumetric pipettes and flasks to prepare 50.0mL of 0.02M Fe(NO3)3 starting from solution 1 (which is your 0.04M Fe(NO3)3 solution from step 2 above). Mix well. Your new solution is solution 2; you have performed a 2-fold dilution to give a 0.02M Fe3+ solution. 4. Use the method of serial dilutions to prepare 50.0mL each of solutions 3, 4, and 5, which are 0.01M, 0.005M and 0.0025M respectively. To make serial dilutions, first dilute part of solution 1 to make solution 2, and then part of solution 2 to make solution 3, etc. Use Eq. 4 in the introduction to calculate the volume of each solution that needs to be diluted to prepare 50.0 mL of the next solution. -5- Summary of Solutions 1 – 5: Solution # 1 2 3 4 5 [Fe3+] 0.04 M 0.02 M 0.01 M 0.005 M 0.0025 M Preparation and Analysis of Equilibrium Mixtures 1. 2. 3. 4. 5. 6. 7. Pipet 5.0mL of solution 1 into test tube B, containing KSCN. Swirl to mix. (Remember, solution 1 was 0.04M before you added it to the SCN- in test tube B; now its volume has doubled and its concentration has halved to 0.02M.) Blank/calibrate the spectrometer (446nm) using solution 2 (0.02M Fe3+) Measure the absorbance for the mixture B at 446nm. Repeat steps 1 – 3, mixing solution 2 and sample C and using solution 3 as a blank. Repeat steps 1 – 3, mixing solution 3 and sample D and using solution 4 as a blank. Repeat steps 1 – 3, mixing solution 4 and sample E and using solution 5 as a blank. The following table may be a helpful guide: Mixture 1 and B 2 and C 3 and D 4 and E CALCULATIONS Blank 2 3 4 5 Determination of molar absorptivity () of FeSCN2+ 1. 2. 3. Calculate the initial concentration of KSCN using the concentrations and volumes of solutions mixed. Assume that all KSCN is converted to FeSCN2+; this gives you the equilibrium concentration of FeSCN2+. Use Beer’s Law to calculate . (Do this in lab and confirm your result with your instructor) Determination of Keq 1. Calculate the concentrations obtained by the serial dilution of the Fe(NO3)3 stock solution; these are the concentrations of solutions 1, 2, 3, 4, and 5. 2. Calculate the initial concentrations of each of the starting mixtures B, C, D, and E. -6- 3. 4. Use Beer’s law and measured absorbances to determine the equilibrium concentration of FeSCN2+. Use reaction stoichiometry to determine the equilibrium concentration of Fe3+ and SCN-: [Fe3+]eq = [Fe3+]initial - [FeSCN2+]eq [SCN-]eq = [SCN-]initial - [FeSCN2+]eq 5. Calculate Keq for each initial concentration and calculate an average Keq (Kav). -7- TABLES and GRAPHS Here are some sample data tables for you to copy into your lab notebook: Determination of FeSCN2+ Absorbance, A Molar Absorptivity, Data Initial Concentrations of Reaction Mixtures Test Volume of Volume of Total Fe3+ initial SCN- initial Tube Fe3+ SCN- added Volume concentration concentration added (mL) in Test (M) (M) (mL) Tube B 5.00 mL 5.00 mL 10.00 mL C 5.00 mL 5.00 mL 10.00 mL D 5.00 mL 5.00 mL 10.00 mL E 5.00 mL 5.00 mL 10.00 mL Test Tube B C D E POST-LAB QUESTIONS 1. 2. Equilibrium Concentrations of Reaction Mixtures Observed Calculated Data Data Absorbance [FeSCN2+]eq [Fe3+]eq [SCN-]eq Keq (measured) (calculated) (calculated) (calculated) (calculated) A mixture is prepared by combining 3.0 mL of 0.0050 M NaSCN (in 0.10 M HNO3) solution with 4.0 mL of 0.0030 M Fe(NO3)3 solution, and 3.0 mL of 0.10 M HNO3 solution. Based on your average Keq value, determine the equilibrium concentrations of Fe3+ , SCN--, and FeSCN2+ in the solution. Consider a competing equilibrium in the system: FeSCN2+ + SCN- Fe(SCN)2+ What error would this competing equilibrium cause in the value of the constant that we are calculating? How would the color, or lack of it, of the Fe(SCN)2+ affect this error? (Hint: Fe(SCN)2+ may or may not interfere with the measurement of FeSCN2+). 3. When making your absorbance measurements, why do you use a blank with a different number than the mixture number? For example, why do you blank mixture E with blank 5? -8- NOTES Strictly speaking, the expression for Keq should be expressed in terms of the activities of the species involved, rather than in terms of their concentrations. The activity of a species may be considered to be its "effective" concentration in a system rather than its actual concentration. The activity of a solute is similar to its concentration, except that it includes interactions between solute particles in the solution. These interactions slightly modify the relative availability of solute particles in equilibrium reactions. For many applications of the equilibrium concept, concentrations are used rather than activities in the expression for Keq, primarily for convenience. However, we must recognize that some error must occur because of this and Keq may not be exactly constant. You will follow that practice in this exercise, using concentrations instead of activities. -9-