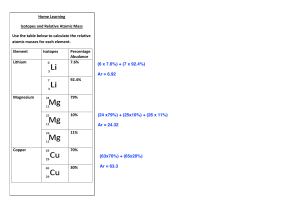
Isotopes and Mass Spectrometry 1. Copper has two naturally occurring isotopes, copper-63 and copper-65. A sample of 250 copper atoms contains 173 atoms with a mass of approximately 63amu. a. How are these isotopes similar? b. How are these isotopes different? c. Use the information above to explain the atomic mass of copper shown on your periodic table. 2. Rhenium consists of two isotopes 185Re and 187Re, in the atomic ratio of 2:3. Calculate the RAM of rhenium. 3. Consider the mass spectrum shown below: a. How many isotopes of element X exist? b. What is the mass of the most abundant isotope? c. What is the mass of the heaviest isotope? d. Estimate the relative atomic mass of element X. Justify your prediction. e. Identify element X. 4. Use the mass spectrum below to calculate the relative atomic mass of lithium. Hint – determine the total rel. intensity and hence % abundance first 5. Deduce the chemical species present in the mass spectrum below. Justify your response. 6. Calcium has two stable isotopes – calcium 40 (39.96amu) and calcium-46 (45.95amu). Determine the % abundance of each isotope. 7. Chlorine-35 (34.969amu) and chlorine-37 (36.966amu) both occur naturally. How many atoms with 20 neutrons would you expect to find in a sample of 500 chlorine atoms? Challenge Question Silicon has three common isotopes – 28Si, 29Si and 30Si. Silicon-29 occurs in 4.67% of atoms. Calculate the % abundance of the other two isotopes.