Isotopes Worksheet
advertisement
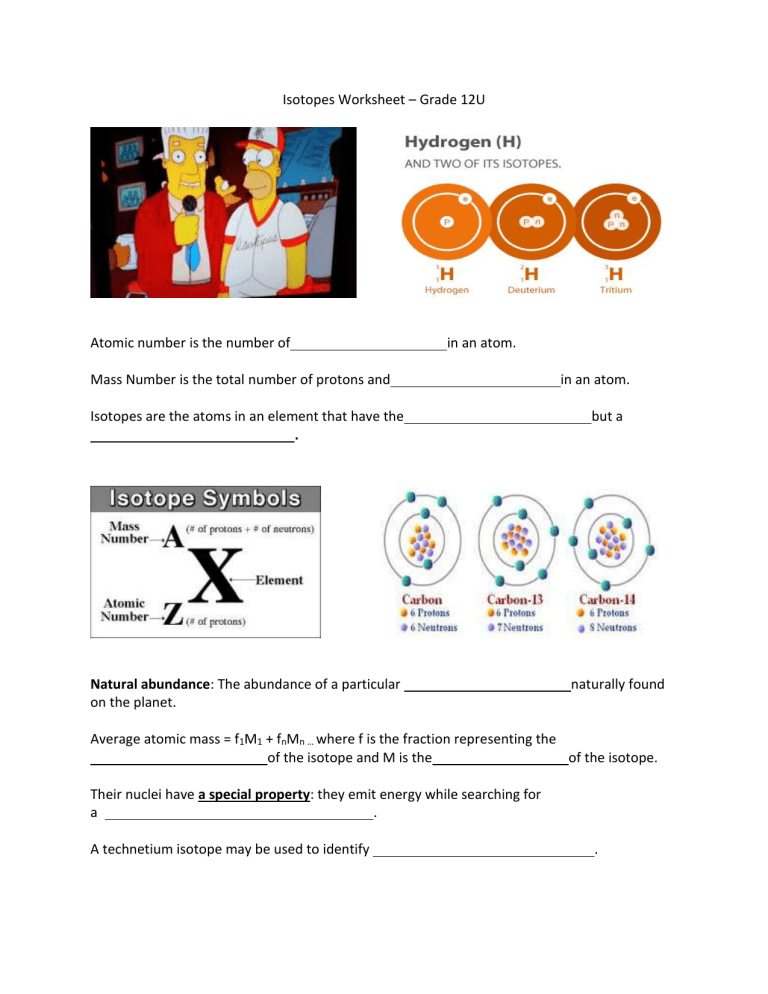
Isotopes Worksheet – Grade 12U Atomic number is the number of in an atom. Mass Number is the total number of protons and Isotopes are the atoms in an element that have the . Natural abundance: The abundance of a particular on the planet. in an atom. but a naturally found Average atomic mass = f1M1 + fnMn … where f is the fraction representing the of the isotope and M is the of the isotope. Their nuclei have a special property: they emit energy while searching for a . A technetium isotope may be used to identify .