Name
advertisement

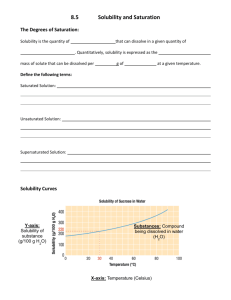
B. Guided Reader – Introduction to Solutions Name: 1. Read about “Homogeneous Mixtures” on page 45. In your own words, describe a solution: ____ 2. Explain why the following can be classified as solutions: a. Air: ____ b. Ocean Water: ____ c. Steel: ____ Characteristics of Solutions 3. Read page 450. An aqueous solution is a specific type of solution. What is it? ____ a. Describe the solute: b. Describe the solvent: c. The ____ dissolves the ____. d. Solvents and solutes may be ____, ____, or ____. 4. Read the last paragraph on page 473. Some chemicals will mix to become solutions and some will not. a. Will salt dissolve in water? ____ Salt is said to be soluble in water. What does soluble mean? ____ i. Give another example: ____is soluble in ____. b. Will rocks dissolve in water? ____Rocks are said to be insoluble in water. What does insoluble mean? ____ i. Give another example: ____is insoluble in ____. 5. It is only appropriate to use the terms miscible and immiscible when talking about the solubility of ____. a. For example, ____and ____are miscible, while ____and ____and immiscible. ________________________________________________________________________________ F. Guided Reader – Solvation in Aqueous Solutions 1. Read page 451. Describe solvation: ____ 2. “____dissolves ____” is the rule used to determine if solvation will occur in a certain solvent. a. Solvent and solute must be “alike” in terms of size and ____. Aqueous Solutions of Ionic Compounds 3. The process of salt dissolving in water is described on page 451. When a crystal of sodium chloride is placed in water, the water molecules ____with the crystal. A water molecule is ____with a partial ____charge on the oxygen atom and partial ____charges on the hydrogen atoms. The ____charged sodium ion will be attracted to and pulled by the ____charged oxygen atom, while the ____charged chloride ion will be attracted to and pulled by the ____charged hydrogen atoms. 4. Draw a picture of the process described in #3 on a separate sheet of paper. Aqueous Solutions of Molecular Compounds 5. Study the diagram to the right in order to describe how sugar dissolves in water. How is it different than salt dissolving in water? ____ 6. Why doesn’t oil dissolve in water? ____ 7. Why do oil and gasoline mix? ____ 8. Use the electronegativity table on page 177 to complete the table below. Bond Electronegativity Difference (show work!) (bigger # minus smaller number) Type of Bond (Non-Polar Covalent, Polar Covalent, or Ionic) NP = less than 0.4 P = 0.5 – 1.7 I = greater than 1.7 Na – F Ag – Cl (Ag is 1.93) (Metal with Nonmetal!) C–F C–O N–H S–H 9. Draw the Lewis structure & determine the shape of each of the following molecules: (on the same paper as you completed #4) H2S CO2 CF4 NH3 10. Using your results from #8 and #9, complete this table. Name Formula Water soluble? Why or why not? Carbon dioxide NaF Silver chloride CF4 Dihydrogen Monosulfide Ammonia 11. What would be able to dissolve the chemicals that water cannot? ____ Would this dissolve all of them? ____ ________________________________________________________________________________ I. Guided Reader – Factors that Affect Solvation Rate Read p. 471 – 476. Then answer the questions below. 1. There are three common ways to increase the rate at which a solute dissolves. List the three ways and describe how each one works. a. ____ because…____ b. ____ because…____ c. ____ because…____ Solubility (p. 472 - 473) 2. Describe solubility: ____ a. For example: ____ grams of sodium chloride will dissolve in 100 g of water at 25ºC. b. Define a saturated solution: ____ c. Define an unsaturated solution: ____ Factors that Affect Solubility (p. 474 - 476) 3. Temperature and Solubility The solubility of most substances ____ as temperature increases. a. This is not true for ____ (their solubility decreases as the solution heats up). b. Define a supersaturated solution: ____ i. Describe how to make a supersaturated solution: ____ 4. What does the graph to the right show? ____ a. What is the solubility of KClO3 at 30.0C? ____ b. What is the solubility of KClO3 at 60.0C? ____ 5. 40.0 g of KCl are added to 100.0 mL of water at 25.0C. Is the solution unsaturated, saturated, or supersaturated? ____ a. At what temperature would all of the KCl dissolve? ____ Now is the solution unsaturated, saturated, or supersaturated? ____ b. Imagine you carefully cool the solution back to 25.0C and all of the KCl remains in solution. Now is the solution unsaturated, saturated, or supersaturated? ____ 6. Describe what is unusual about cesium sulfate. ____ What other substances have this solubility trend? ____ 7. 100.0 g of CaCl2 are added to a beaker filled with 200.0 g of water. Is the solution unsaturated, saturated, or supersaturated? (consider all temps) ____ Pressure and Solubility (p. 476) 8. Pressure only affects the solubility of ____ 9. Gas solubility increases as the partial pressure of the gas above the solution ____. 10. Describe how this principle is used to keep soda “fizzy.” ____