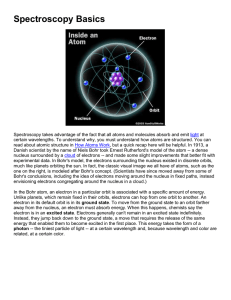
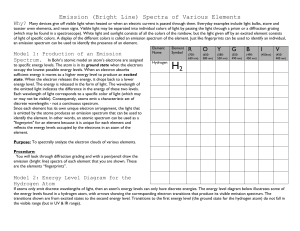
Sixteen – Energy Levels in Atoms
advertisement

Sixteen - Energy Levels in Atoms 1. define ground state as the lowest energy state that can be occupied by an electron in an atom 2. explain that electrons need energy to move up a level, which can only be received by a photon of a specific energy (from radiation of a certain frequency). to move down a level, the electron must emit a photon 3. use the equation ℎ𝑓 = 𝐸1 − 𝐸2 where E1 is the energy of the level the electron leaves and E2 is the energy of the level the electron enters 4. understand that an energy level of 0 means that the electron is free from the atom 5. understand that the energy of an electron is quantised in that only specific photons can move the electron up 6. define a spectrum as a collection of waves with a range of frequencies in the visible spectrum 7. define spectral line as a line relating to a specific frequency of light either missing from an absorption spectra or present in an emission spectra 8. explain that liquids and solids cannot be used well to produce emission and absorption spectra because their atoms are too close and their electrons interact with neighbouring atoms, which alters the energy level diagram making it too complex 8. explain that isolated atoms in gases produce simple line spectra and are evidence for the existence of discrete energy levels: -Gas (isolated) atoms can only absorb or emit light photons of specific frequencies ( that are characteristic of a given element).This behaviour is evident from their absorption and emission spectra. In an absorption spectra specific frequencies (colours) are missing - leaving a fine dark line on an otherwise continuous spectrum, showing which photons have been absorbed. An emission spectrum shows that individual gas atoms only emit photons (light) of specific frequencies - showing as a set of individual, fine bright coloured lines that correspond exactly with the absorption lines. Assuming that atomic electrons are responsible for absorbing photon energy .. and emitting it - the existence of specific spectral lines, that coincide in both the absorption and emission spectra, is evidence that electrons within an atom are only permitted a certain set of energy values (levels)... and they can only move between these specific permitted levels as they gain or lose energy (by absorbing or emitting photons) 9. explain that emission spectra involve hot gases where electrons have moved to higher levels, and as they fall back down to ground state they emit photons producing emission spectra. each line of the emission spectra corresponds to a particular wavelength of light emitted by the source 10. explain that absorption spectrum involve cool gases that remove certain frequencies/wavelengths of light from a continuous spectrum, because at low temperatures the electrons will be at their ground states, and photons of certain frequencies are absorbed by the electrons to excite them to a higher energy level. these wavelengths are now missing from the continuous spectrum produced by the source