Spectroscopy
advertisement

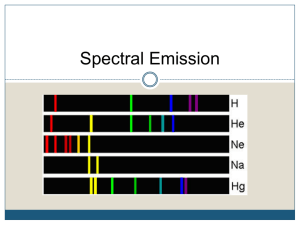
Spectroscopy • spectroscopy: breaking up light into its component colors to study how atoms and light interact • dispersion: spreading out of white light • spectrum: the spectra of colors produced by sending white light through a prism • spectroscope: instrument used to see spectra (ex. in class) Types of Spectra • continuous spectrum: a spectrum with no breaks. A continuum of unmixed shades of color. • spectral (emission) line: a well defined, thin line of one specific color • emission-line spectrum: thin lines of specific colors against a dark background • absorption line: a dark line on a continuous spectrum marking a lack of intensity of a specific color (not necessarily a complete absence of that color) • absorption-line (dark-line) spectrum: dark lines of missing color against a continuous background (ex: solar spectrum) More on spectra • polarized light: all light propagating (waving) in the same direction. A spectroscope polarizes light prior to its dispersion. This ensures that all spectral lines are vertical and parallel. • uniform speed of light: light travels at same speed through the same medium. Light travels fastest through a vacuum. Kirchoff’s Rules • 1. A hot, opaque solid liquid, or highly compressed gas emits a continuous spectrum. Example: filament of a incandescent light bulb • 2. emission-line spectrum => A hot, transparent gas produces a spectrum of bright lines (emission lines). The number and colors of these lines depend on which elements are present in the gas. Example: a neon sign • 3. absorption-line spectrum => If a continuous spectrum (from a hot opaque solid, liquid, or gas) passes through a gas at a lower temperature, the cooler gas causes the appearance of dark lines (absorption lines). Their colors and numbers depend on the elements in the cool gas. (99% of stars have this spectrum) Example: sunlight • Show me Kirchoff’s Rules Spectral Summary • every element /isotope displays a unique arrangement of lines in its spectra • emission and absorption lines for the same element are in identical positions Balmer Series • The set of hydrogen absorption or emission lines that lie in the visible part of the spectrum, the first of which is the H-α line (in the red visible region) • This very orderly pattern of spectral lines led scientists to look for a cause in the internal structure of the atom Particle nature of light • quanta: “chunks” of light energy (photons) emitted by radiating matter • photon: a quanta (piece) of light • photoelectric effect: Ephoton = hf = hc / λ • • • • • (since f = c / λ) E = energy of the photon f = frequency of the photon h= Planck's constant (6.63 x 10-34 J·s) λ: wavelength of photon c : speed of light (3 x 108 m / s in a vacuum) Bohr’s atomic model • explains and predicts why absorption and emission of photons take place. • Emission and absorption of light is due to transitions between electron energy levels • energy levels: electrons have a large number of levels with specific energies (stair step example) • ground state: the 1st (lowest) energy level. When the electron occupies this level, the atom is at its minimum energy. • the higher the total energy of the atom, the closer the orbits (or electron energy levels) are to each other. Excitation & De-excitation Model of atomic energy levels • excitation: moving an electron to a higher level (yields an absorption line) – 2 methods of excitation: collision with another atom or absorption of a specific photon with sufficient energy. • de-excitation: the electron descends to a lower level (yields an emission line) – The electron loses energy and this exact energy is given off by the emission of a photon. • electrons only move to exact energy levels (don't take half-steps) • Ionization: if an atom gains enough energy, the electron flies away from (escapes) the nucleus. It is no longer bound to the atom. • Ionization energy: the amount of energy needed to ionize an electron. Balmer series explained • Emission or absorption lines arising from transitions between the 2nd level and all others (except the 1st) • Photons at visible wavelengths Hydrogen-specific series • • • 5 4 5 4 3 3 2 2 1 1 Lyman series • Emission or absorption • lines arising from transitions between the 1st level and all others. Photons at ultraviolet wavelengths • Balmer series Emission or absorption lines arising from transitions between the 2nd level and all others (except the first). Photons at visible wavelengths • • • Paschen series Emission or absorption lines arising from transitions between the 3rd level and all others (except the first and second). Photons at infrared wavelengths Balmer Thermometer • Balmer Lines: spectral lines of H caused by bound-bound transitions to and from the 2nd energy level – – – – from 3->2: red line from 4->2: blue line from 5->2: violet line from 6->2: violet line • If a star is too cool; few electrons are excited to or above the 2nd energy level (3000K; 6000° F) • If a star is too hot; most electrons are excited above the 2nd level • "In between" stars have the strongest Balmer Lines (Spectral Class: A) Spectral Classification • • • -Original Method: – depended on Balmer line strength only; – no understanding of relationship to temperature; – classified from strongest (A) to weakest (Z) Balmer lines But, weak Balmer lines can be from hot and cool stars... Modern Stellar Spectra Sequence: reorganized classes by temperature: (hot) O b l u e / w h i t e • Also a color scale! B b l u e / w h i t e A w h i t e / b l u e F w h i t e / y e l l o w G y e l l o w K o r a n g e M r e d (cool) Spectral sub-classes • Spectral subclasses. (0-9) differ between intensities of specific absorption lines • ex: (hotter) G0, G1, G2... K0, K1 (cooler) • Strengths of Balmer lines suggest differences in stellar spectra & reflect differences in temperatures • (the hotter the) Temperature --> (the more) Collisions ---> (the more) Ionization