Spectra Atomic Energy Levels Solutions
advertisement

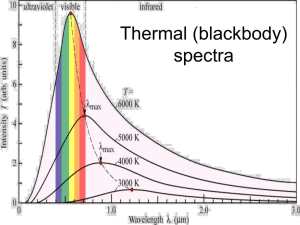
Le Fevre High School SACE Stage 2 Physics Spectra and Atomic Energy Levels Solutions 1. Continuous Emission Spectra Spectra given out by hot solids or liquids. They consist of a continuous range of frequencies given out with varying intensities. Both the intensity of each frequency and the frequency which is most intense very with temperature. These spectra are dependent only on temperature and are independent of the type of solid or liquid which is emitting the radiation. Line Emission Spectra Spectra given out by hot atomic gases. They consist of discrete frequencies of radiation each given out with different intensity. The actual frequencies given out are characteristic of the type of element giving out the radiation. Both the number of lines and the intensity of them are dependent on temperature. Line Absorption Spectra Spectra produced when white light from a continuous emission spectra is passed through a large volume of monatomic gas. The "absorption" lines are characteristic of the type of gas through which the light is passed and the Number of lines dependents on the temperature of this gas. The lines are a subset of the lines emitted by the gas if it is heated so that it emits a line emission spectrum. Emission spectra are those produced when a substance gives out radiation (glows in the dark is a good test in the visible region). Absorption spectra are those produced when radiation from an incandescent source is absorbed and the re-radiated in all directions. The intensity of the lines or bands in the spectrum for these frequencies is significantly reduced and so these parts of the spectrum appear dark. 2. Spectra are characteristic of each element. Because emission and absorption spectra depend on the electronic structure of an atom (or molecule) and this is unique for each element. (The binding energies of each orbit of an atom are a result of the size and charge of the nucleus.) 3. Fraunhofer lines are the dark absorption lines occurring in the continuous emission spectrum of the sun. These lines appear dark because some of the light from the sun is absorbed by atoms in the hot, outer atmosphere of the sun. This light is then re-radiated in all directions with only a small fraction of the light coming in the direction of the earth. By contrast with the unabsorbed light this re-radiated light is low in intensity and so the lines appear dark. 4. (a) ground state i.e. state of lowest energy (Highest binding energy) (b) n = 4 to n = 2 with E = 2.51 eV (c) Wave length of n = 4 to n = 2 hc 6.63 x 10 34 x 3 x 10 8 is = = E 2.51 x1.6 x 10 19 = 4.93 x 10-7 m (or 493 nm) 5. "The first excited state is at 10.2 eV" means that the n = 2 energy state is 10.2 eV higher in energy than the n = 1 state. Hence the electron is the ground state must absorb 10.2 eV of energy if it is to jump into the next highest state. Le Fevre High School "The ionisation energy is 13.6 eV" means that the energy required to completely remove the ground state electron is 13.6 eV. (a). Photon is not absorbed, minimum energy for absorption is 10.2 eV. (photon scatters) (b). Photon is not absorbed. No transition corresponds to this energy exactly. (photon scatters) (c). Photon may be absorbed. If it is the absorbing electron will gain 14.6 eV of energy so will be freed from the atom. The free electron will have kinetic energy 14.6 - 13.6 = 1 eV. The atom will be left in an ionised state. 6. . E2 - E1 = hf = h 6.63 x 10 34 x 3 x 10 8 c = 656.3 x 10 9 = 3.0 x 10-19 J 7. n=4 n=3 (a) B1 B2 B3 n=2 L2 L1 n=1 i.e. n = 5 and n = 2 (b) From energy level diagram E for L2 - E for L1 = E for B1 hfL2 - hf L1 = hfB1 fL2 - fL1 = fB1