Diffusion and Osmosis lab handout
advertisement

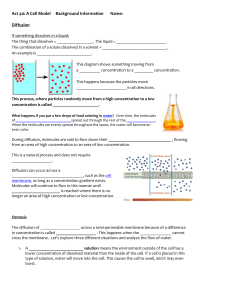
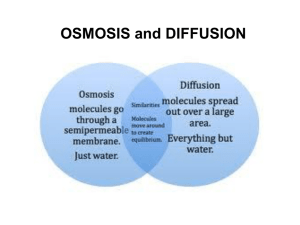
Name:_______________________ Section:__________________ Diffusion and Osmosis Objectives: Be able to do the following: 1. Define these terms in writing: diffusion, osmosis, equilibrium, relative concentration, solvent, semi-permeable membrane, concentration gradient, solute, solution, hypertonic, hypotonic, isotonic 2. Explain why a particular material diffuses in a particular direction 3. Determine the net direction of diffusion 4. Differentiate between diffusion and osmosis 5. Describe the influence that varying the concentration of solute and solvent has on the rate of osmosis Introduction: Diffusion Although you may not know what diffusion is, you have experienced the process. Can you remember walking into the front door of your home and smelling a pleasant aroma coming from the kitchen? It was diffusion of molecules from the kitchen to the front door of the house that allowed you to detect the odors. Diffusion is defined as the net movement of molecules from an area of greater concentration to an area of lesser concentration until the concentration everywhere is the same. To better understand how diffusion works, let’s consider some information about molecular activity. The molecules in a gas, a liquid, or a solid are in constant motion because of their kinetic energy. Moving molecules are constantly colliding with each other. These collisions cause the molecules to move randomly. The higher the concentration of molecules in one region, the greater the number of collisions. Some molecules are propelled into the less concentrated area and others are propelled into the more concentrated area. Over time, however, there will be more collisions in the highly concentrated area, resulting in more molecules being propelled into the less concentrated area. Thus, the net movement of molecules is always from more tightly packed areas too less tightly packed areas. Diffusion occurs when there is a difference in concentration from one region to another or from one side of a membrane to another. This unequal distribution of molecules is called a concentration gradient. When the molecules become uniformly distributed they have reached equilibrium. The process of diffusion occurs in both living and nonliving systems. Biologically speaking, diffusion is responsible for the movement of a large number of substances, such as gases and small uncharged molecules, into and out of living cells. The net direction of diffusion is always from where there were originally more molecules to where there are fewer. Imaging that your instructor opens a bottle of ammonia in a corner of the room. The bottle would have the highest concentration of ammonia molecules in the room; the individual ammonia molecules would move from this are of highest concentration to where they are less concentrated. Although you could not actually see this happening, ammonia molecules would leave the bottle and move throughout the air in the room because of molecular movement. You could detect this by the odor of the ammonia. If you compare the relative number of ammonia molecules in the bottle to those dispersed in the room, you would be dealing with what is called relative concentration. Relative concentration compares the amount of a substance in two separate locations. Whenever there is a difference in concentrations of a substance, you can predict the direction n that most of the molecules will move. You can predict that when the bottle is first opened, ammonia molecules will move from the area of higher concentration (the bottle) to the region of lower concentration (the air in the room). Soon, however, the molecules of ammonia will mix with the air molecules in the room. Because the ammonia molecules are moving randomly, some of them will move from the air back into the bottle. As long as there is a higher concentration of ammonia molecules in the bottle, more of them move out of the bottle than move in. When the number of molecules moving out of the bottle equal the number of molecules moving into the bottle equilibrium has been reached. Another example of diffusion is sugar dissolving in water. When sugar molecules are water molecules mix a solution is created. A solution is any mixture where different types of molecules are dispersed throughout the system. In the sugar solution the sugar is referred to as the solute and the water is referred to as the solvent. Procedure: Diffusion 1. Sergeant Stinky Man has knocked over his bottle of pungent cologne in the barracks. Draw arrows in the picture below to signify how the scent would diffuse from the spilled bottle when it was initially spilled. Remember to draw arrows in the direction of all possible molecule diffusions. You will have more arrows moving in one direction than the other. Introduction: Osmosis A differentially permeable membrane is a thin sheet of material that selectively allows certain molecules to cross but prevents others from crossing. The membrane in the picture below is permeable to water molecules only. Water molecules may freely diffuse across the membrane, but other types of molecules cannot. The diffusion of water across a differentially permeable membrane is called osmosis. On each side of the differentially permeable membrane below is a sugar solution. A solution is characterized by the dissolved substance called the solute. Sugar in this example is the solute. The substance which the solute is dissolved is called the solvent. The solution that has the higher concentration of solute compared to the other solution is referred to as hypertonic. The solution that has the lower concentration of solute compared to the other solution is referred to as hypotonic. Solutions that have equal concentration are referred to as isotonic. There is a net movement from a hypertonic solution to a hypotonic solution. There is a net movement of water in the below situation. It is going to be from a place of higher concentration (hypertonic) to a place of lower concentration (hypotonic). This movement will continue until the solutions separate by the semipermeable membrane are isotonic. Procedure: Diffusion 1. Take four pieces of the precut dialysis tubing and place them in distilled water to moisten them. 2. Take four cups and label one 0% sucrose, one 10% sucrose, one 20% sucrose, and one 40% sucrose. 3. Tie a knot close to one end of a piece of tubing, and then gently open the other end and pour in 10 ml, of a particular solution (0% sucrose, 10% sucrose, one sucrose, 40% sucrose) 4.Work out bubbles and tie the bag so that it is limp and not stuffed full of fluid 5. Rinse the bag in the large finger bowl (it contains distilled water) to remove any sugar solution that may be on the outside, pat them dry, and place them in the appropriate labeled cups 6. Weigh each of the bags, record the weights, and replace them in their appropriate cups 7. Fill each of the cups with 200 ml of distilled water, Immersing each bags 8. At 15 minute intervals dry and weigh each bag, and record the weights 9. Calculate the total weight change and the percent weight change over the entire time period 10. Graph the raw data and interpret the results Record your data: Diffusion Bag: (10 ml) Beaker: (200 ml) Weight of bag in grams at 15 minute intervals 0 15 30 45 60 Total weight change % weight change Bag: dist. water Beaker: dist. water Bag: 10% sucrose Beaker: dist. water Bag: 20% sucrose Beaker: dist. water Bag: 30% sucrose Beaker: dist. water Analyze your data: Diffusion Graph all of your raw data point for each of your bags. Label the x-axis for the time in minutes and the y-axis for weight in grams. Post lab questions: Diffusion and Osmosis For questions 1 - 6 use this scenario: Two solutions in a container are separated by a semipermeable membrane. On the left side is a 30% chloride solution (in water). On the right side is a 10% chloride solution (in water). The semipermeable membrane is permeable only to water. 1. What is the percentage of solute in the left side of the container? 2. What is the percentage of the solvent in the left side of the container? 3. What is the percentage of solute in the right side of the container? 4. What is the percentage of solvent in the right side of the container? 5. Where is the water in the higher concentration, the left side or the right side? 6. In what direction would be the net movement of the water molecules? 7. In your osmosis experiment. In a perfectly tied and unbroken bag, should we see evidence of sugar molecules passing through the “membrane”? Qualify your answer in terms of how differential permeability operates. 8. From the graph made of your experimental results, what can you conclude about the movement of water during your experiment? 9. From the graph made of your experimental results, what can you conclude is the effect of the varying sucrose concentrations during your experiment?