Diffusion and Osmosis.ppt - Cardinal Newman High School
advertisement
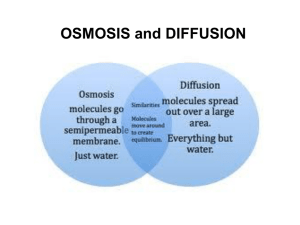
Diffusion and Osmosis Diffusion • Particles in a liquid or gas spread out… • … from regions of high concentration… • … to regions of low concentration… • …until the particles are evenly spread out. Dissolving KMnO4 crystal • The difference between the regions of high concentration and low concentration is called the concentration gradient • The steeper the concentration gradient, the faster diffusion takes place High concentration gradient Fast rate of diffusion Low concentration gradient Slow rate of diffusion • Diffusion occurs because the particles in gases and liquids are moving. Dissolving substances in water • The molecules in liquid water are constantly moving • When water molecules bump into particles of a soluble substance, they stick to them Free moving water molecules Sugar molecules in sugar lump • When the water molecules move away… … they carry particles of the solute with them • Adding a solute to water reduces the amount of free water molecules Free water molecules Solute molecule Partially-Permeable Membranes • A partially-permeable membrane will allow certain molecules to pass through it, but not others. • Generally, small particles can pass through… Partially permeable membrane …but large particles cannot More free water molecules on this side of membrane Partially-permeable membrane Water-solute particle is too large to pass through membrane Free water molecules diffuse in this direction Osmosis • Osmosis is the diffusion of free water molecules… •… from a region of high concentration of free water molecules… • … to a region of low concentration of free water molecules… • …across a partially-permeable membrane… • …until they are evenly spread out.