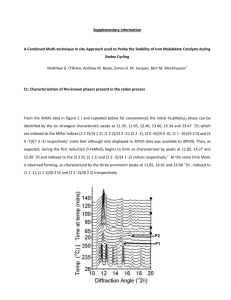
handout p. 1-5 - Bryn Mawr School Faculty Web Pages
advertisement

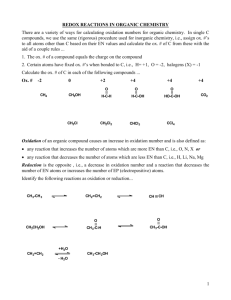
OXIDATION NUMBERS Review 1 When an atom becomes involved in a bond (and thus forms a compound) the atom is either ______________________________ or _____________________________. 2. When an atom loses electrons, it has been ____________________________________; when an atom gains electrons, it has been ____________________________________. 3. Do you remember burning the magnesium ribbon in our last lab? Use redox to analyze this reaction. 3. “The only kind of reaction in which an atom truly loses an electron, and another truly gains one is one that results in an ____________________ bond.” BUT: The reaction which destroyed the Hindenburg was also a redox reaction! 2H2 + O2 → 2H2O These are molecules, not ionic compounds. How can they have oxidation numbers if they don’t have ions? Reminder: The charges on the ions in an ionic compound are whole numbers (1+, 2+ 3+, 1-, 2-, 3-…) because electrons are ________________________________________ in an ionic compound. In a molecular compound, there are either partial charges (δ+ δ-) on the atoms or no charges (0) because the atoms ______________________ _____________________________the electrons. Concepts of Oxidation numbers 4. The oxidation numbers in molecular compounds are not actual charges (like they are in ionic compounds); they are assigned values to keep track of the ___________________. They’re BOOKKEEPING!! The oxidation numbers in molecular compounds are WHOLE numbers, even though the actual charges are partial or zero. PARTIAL In a molecular bond, the atom with the greater ______________________________ is assigned the negative oxidation number, and the other atom, which has the lesser ______________________________ is assigned a positive charge. 5. A pure element that is all by itself always has an oxidation number of _______________ 6. The following elements always have the same oxidation number: ________________________________ always have an oxidation number of +1. __________________________________ always have an oxidation number of +2 The oxidation number of aluminum is always _________________. Fluorine always has an oxidation number of ____________________. 7. The following elements often have this oxidation number, with some exceptions: Hydrogen is usually _________________; however, when it is combined with a metal, it is ___________. Oxygen is __________________, except in peroxide it is ________________. 8. In binary compounds, halogens are generally _____________. 9. In a compound, the sum of the oxidation numbers is ________________. 10. In determining oxidation numbers in binary compounds, start with the element that is more electronegative. This will normally be the LAST/SECOND* element in the formula. Common Exceptions: ________________________ and __________________________ * Screencast says first element; it’s wrong! Practice in Determining Oxidation Numbers 11. Determine the oxidation numbers for the elements in each of the substances below. A) Phosphorous B) PCl3 C) PCl5 D) CO E) CO2 F) H2O G) NH3 H) LiNO3 I) KMnO4 J) SO42- K) PO31- L) H2Cr2O7 M) NH4ClO4 N) HClO3 O) Ca(ClO2)2 P) Bleach – (NaOCl) Q) Cl2 R) AsCl3 S) acetylene (C2H2) T) hydrogen peroxide (H2O2) U) sodium hydride (NaH) BALANCING REDOX EQUATIONS Oxidation numbers can be used to balance difficult redox equations. For example: the space shuttle is lifted off the earth by this tough redox reaction: NH4ClO4 + Al → Al2O3 + HCl + N2 + H2O This would take forever to balance, based on the method we have used to date (which is called _______________________________________) However, the Law of Conservation of Mass says that “matter is neither created nor destroyed in a chemical reaction – just rearranged.” Electrons are matter, so – however many electrons one substance loses is the same number that another substance must gain. This fact can be used to balance tough redox reactions. This method is called the ___________________________________________________ method. Example: __HNO3 + __H2S → __S + __NO + __H2O Practice 13. __H2Cr2O7 + __HI + __HCl → __CrCl3 + __I2 + __H2O 14. __KCl + __KMnO4 + __H2O → __Cl2 + __MnO2 + __KOH ______________________________________________________ 15. __NH3 + __NO2 → __N2 + __H2O Electrochemistry 16. Electrochemistry is the study of the relationship between _________________________ ________________________________ and ____________________________________ Put more precisely: electrochemistry is the study of the redox processes by which ______________________________ energy is converted to _______________________ energy and vice versa. “Electrochemical processes are useful in ______________________, and critical in _________________________________ functioning.” An example of the importance of electrochemistry in our bodies: Every cell has a voltage across its plasma membrane. (Voltage is electric potential energy that arises from the separation of opposite charges.) This is particularly important in nerve cells, because it is changes in this potential that allows electrical signals to flow along our nerve cells. 17. A battery is a type of electrochemical cell, also known as a ___________________________________ or ______________________________ cell. This type of cell involves a spontaneous chemical reaction, in which chemical energy is converted to electrical energy. “A spontaneous process is a chemical or physical change that occurs with _________________________________________________________.” Example Copper wire is put into a solution of silver nitrate. 18. We can tell that copper metal + silver nitrate is a spontaneous reaction, and that the opposite reaction (silver metal + copper nitrate) is non-spontaneous because we actually tried these reactions. How can we tell if these reactions are spontaneous or non-spontaneous without physically doing them? There are 2 different methods we can use: A) Non-quantitative method: the_________________________________(see appendix) B) Quantitative method for determining if redox reactions are spontaneous or not, and also for calculating the E0 for a reaction/electrochemical cell, (e.g., a battery) This method uses Standard Reduction Potentials. A reduction potential is a quantitative measurement for the tendency of a substance to be reduced – i.e., how much it “wants” to ________________ electrons. Since reduction can only occur when there is also oxidation, the reduction potential of a substance must be measured versus another substance. The standard reference potential is versus ___________________________. 2H1+ + 2e1- → H20 E0 = 0.000 volts Standard conditions ( 0 ) are 1 molar for ____________, and 1 atmosphere for ________ The sum of the potentials of the two half reactions equals the potential of the electrochemical cell, E0cell . E0oxidation + E0reduction = E0cell If E0cell > 0, the reaction in the electrochemical cell is _____________________. If E0cell < 0, the reaction in the electrochemical cell is _____________________. If E0cell = 0, the reaction in the electrochemical cell is at equilibrium. Using the table on the next page, calculate the E0cell and state if the reaction is spontaneous or not. NOTE: For the half reaction that is an oxidation, the potential will have the opposite sign from the reduction potential. Also, it doesn’t matter if a half reaction is multiplied by a coefficient; don’t multiply E0ox or E0red by the coefficient. Example 1 E0ox Sn + Cu2+ → Sn2+ + E0oxidation + E0reduction = E0cell = Example 2 2Fe + 3Ca(NO3)2 → 2Fe(NO3)3 + 3Ca E0ox E0oxidation + E0reduction = E0cell E0red = Practice in calculating E0cell and determining if cell is spontaneous or not A) Mg + Pb2+ → Pb + Mg2+ B) 2Al + 3CoCl2 → 3Co + 2AlCl3 E0red The structure of an electrochemical cell 19. When we dipped Cu into AgNO3, the reaction was spontaneous; however, it didn’t produce electricity for us to use, like a battery does. “Electricity involves the flow of _____________________. If we want to derive electrical energy from this reaction, we must force the electrons that leave the ________________________ to flow through a wire in order to reach the __________________________. In an electrochemical cell, the half-reactions are performed in separate ________________________. An ______________________ ( a strip of metal that participates in the reaction) is placed in each half reaction vessel. The electrode in the oxidation vessel is called the __________________; the electrode in the reduction vessel is called the __________________ How can you remember this? Below is a picture of an electrochemical cell. The voltage the cell puts out is theoretically _____________; however, because of the resistance in the ____________ It is actually less. The function of the salt bridge is to prevent ___________________ from building up in either vessel. Over time, the electrodes will change in weight. The __________________ will get heavier, and the ____________________ will get lighter. The voltage will decrease over time; when E0cell becomes zero, the redox reaction is at _______________________, and the battery is dead. Electrolysis It is possible for a non-spontaneous reaction to occur; however you must __________________ to happen. The method used to force a non-spontaneous redox reaction to happen is called _________________________. In this, you put ___________________________ energy into the non-spontaneous reaction, which forces it to occur; thus, you are converting _________________________ energy into _______________________ energy. For example, in industry, putting electricity through molten NaCl produces sodium metal and chlorine gas. _____________________________ is another example; here one metal is “plated” onto another. Everyday examples of this are silverware that is silver-plated and chrome-plated car bumpers. APPENDIX The activity series is a non-quantitative method for determining if a redox reaction is spontaneous or nonspontaneous. Whichever metal is higher on the activity series “wants” to be oxidized more than the one that is lower. Whichever halogen is higher on the activity series “wants” to be reduced more than the one that is lower. Fluorine Chlorine Bromine Iodine Examples: Al + FeCl3 → Al0 + Fe3+ → spontaneous Reactivity Series for Halogens Fe + AlCl3 → Fe0 + Al3+ → non-spontaneous Sn + HCl → Sn0 + H1+ → spontaneous Ag + HCl → Ag0 + H1+ → non-spontaneous F2 + LiCl → F0 + Cl1- → spontaneous Cl2 + LiF → Cl0 + F1- → non- spontaneous Practice using the Activity Series Determine if the following reactions will happen spontaneously or not. A) Cu + ZnCl2 → ____________________ B) Zn + CuCl2 → ___________________ C) Ca + Pb(NO3) → ____________________ D) Pb + Ca(NO3)2 → __________________ E) Br2 + NaCl → ____________________ F) Cl2 + NaBr → ___________________ G) LiBr + F2 → ____________________ H) Br2 + LiF → ___________________ Extra example of calculating E0 and spontaneity 2Mn2+ + 8H2O + 10Hg2+ E0ox Mn2+ + 4 H2O E0red Hg2+ → Hg22+ → 2MnO41- + 16H1+ + 5Hg22+ → MnO41- + 8H1+- E0 = (-1.507) E0 = (+0.7973) E0oxidation + E0reduction = E0cell = (-1.507) + (+0.7973) = (-0.587 V) non-spontaneous Practice 2SO42- + Co2+ → Co + S2O82--