3-methyl-3-buten-1-ol Tutorial
advertisement

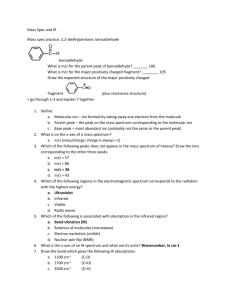
Tutorials 3-methyl-3-buten-1-ol IR spectroscopy is all about identifying the functional groups of a chemical compound. Recall that a functional group is a particular group of atoms in a compound with a specific connectivity pattern which is responsible for the characteristic reactions in that compound. Common examples of functional groups are alkanes, alkenes, alkynes, and aromatic rings. If we know the name of the compound, we can identify its structure and functional groups. Which of the following structures identifies 3-methyl-3-buten-1-ol? 3-4 options for the structure of this compound (based on functional groups, NOT position) Looking at the name 3-methyl-3-buten-1-ol, we identify several functional groups: Buten tells us there is a Carbon double bond, or an alkene in this compound. The methyl indicates there is at least one unsaturated carbon (that is with no multiple bonds) in the compound, an alkane functional group. Finally, the –ol indicates that there is an alcohol (an O-H functional group) present in the compound. Now, suppose that you were not given the name of this compound, only an unknown substance. How could you figure out its functional groups? Chemists use Infrared Spectroscopy to determine functional groups of unknown substances. Many molecules absorb infrared light, causing the bonds in the molecule to bend and stretch. The IR spectrum of a sample examines to what degree a compound absorbs infrared light of different wavelengths. Where the sample absorbs IR light, a peak is produced in the IR spectrum. Because functional groups in a molecule absorb IR light at specific wavelengths, chemists can identify the functional groups in a molecule by analyzing the various peaks in a molecule’s IR spectrum. (What was here originally: Where there is low transmittance (or, from another perspective, high absorbance) the IR spectrum is said to peak. Examining an IR spectrum’s peaks yields much about the chemical’s structure.) Spectrum with dashed line which appears when button is clicked. Show Regions button Consider the IR spectrum of 3-methyl-3-buten1-ol. When looking at the spectrum, chemists divide it into 2 regions. The first region (for wave numbers greater than 1450 cm-1) is called the Functional Group Region and examines the primary functional groups and stretching vibrations. The second region (for wave numbers less than 1450 cm-1) looks at additional data about the compounds stretching and bending vibrations. This is called the Fingerprint Region. We will focus on the Functional group region. QUESTION Look in the Functional Group Region. How many peaks do you see? 4, 5, 6, 7 There are 5 peaks. Each peak tells us something different. (Spectrum with 5 peaks highlighted and numbered) Firstly, consider any peaks around 3000 cm-1. These examine C-H stretch bonds. (zoomed in empty spectrum with shaded regions from 2800-3000, 3000-3100, and 3300, labeled with sketches) QUESTION: Based on the data above, which type(s) of C-H bonds could be present in this compound? Check all that apply: Saturated Carbons Alkene Carbons Alkyne Carbons Aromatic Carbons Saturated carbons (or alkanes) are present because of peak (1). Peak (2) tells us there is either an alkene or an aromatic present. However, there is nothing at 3300, so there is no alkyne in this compound. (Spectrum with colour-coded, numbered peaks highlighted) To determine whether or not peak (2) is an alkene or an aromatic, we must look at secondary peaks in both the functional group and fingerprint regions. Alkenes will have additional peaks at 1600-1670 cm-1 and 675-990 cm-1, while aromatics have additional peaks at 14501600 cm-1 and 690-885 cm-1. They may also have small peaks the 1600-2000 cm-1 region. QUESTION: Based on this information, does our compound contain an alkene or an aromatic? Alkene Aromatic The compound contains an alkene. The peak around 3000 indicates a stretching in the C-H bonds, while the 1600-1670 is a stretch in the double bond, and the early peak is an out of the plane bend in the C-H bonds. (graphics of stretching/bending with peaks highlighted) The next step is to look for C = O bonds. These are marked by intense peaks in the 1600-1800 cm-1 region. Are there any C = O bonds in this compound? Yes No No. While there is a peak in that region, it is not intense enough to be a C=O bond. It is instead the C=C bond mentioned earlier. (graph with lack of bond highlighted) The final stage is to look for either alcohols (O-H bonds) or amines (N-H bonds). If either of these are present, there will be a peak in the 33003650 cm-1 region. QUESTION: Are there any O-H or N-H bonds present in the compound? There is a peak present. Because of this, we must note the phase of the sample (in this case, the sample is a gas). To determine if it is an O-H or an N-H stretch, we must first note the position of the peak. (graph of peak highlighted) QUESTION: At approximately what wavenumber is the peak located? 3300-3500 cm-1 3550-3700 cm-1 (Graph of spectrum with peak highlighted) Because the peak is located at a higher wavenumber in this region, it is likely an alcohol. (Graph of empty spectrums and regions in it, also fingerprint region.) However, we can confirm that this is an O-H bond by looking at the Fingerprint Region. Alcohols have an additional peak at 1000-1300 because of the C-O bond. Similarly, amines have a peak at from the C-N bond at 1030-1230. QUESTION: Click on the peak which confirms that there is an alcohol. Conclusion From this IR spectrum, we have determined that 3-methyl-3-buten-1-ol contains at least one of each of the following functional groups: Alkane, Alkene, and Alcohol. Click the “Try another example” button to walk through another compound, or click the “Samples” button to try some compounds on your own.