and s
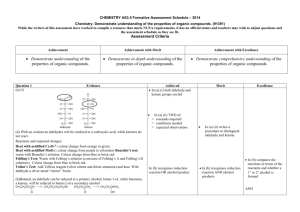
advertisement

Chiral molecules A chiral (from the Greek cheir, hand) molecule has the same relationship with its mirror image as the right hand with the left If an improper rotation axis Sn is present the molecule cannot be chiral. This holds clearly for S1 (plane of symmetry) and S2 (centre). Is 12 chiral? Are the following species polar? Chiral? C6H5Cl, CHCl3 , 1- or 2– substituted norbornane, 2-methylpentane…. poly-3-(2-methylbutyl)thiophene) PTFE c = 19.5 Å Molecules with more than one chiral centre 2,3-dichloropentane has two asymmetric C and two diastereoisomers. For each diasteroisomer there is in this case a pair of enantiomers. Diastereoisomers are not mirror images and have different physical and chemical properties mirror mirror R,S S,R non superimposable enantiomers, erythro S,S R,R non superimposable enantiomers, threo In Fischer projections the similar groups are: on the same side erythro pair on opposite sides threo pair Molecules with more chiral centres (2) 2,3-dichlorobutane Also 2,3-dichlorobutane two asymmetric C and two optically active diastereoisomers, but the three groups, on each of the two C*, are identical: mirror S,S mirror R,R non superimposable enantiomers S,R R,S superimposable meso compound Molecules with more than one chiral centre (3) Meso compounds are achiral: there is an internal compensation among the chiral centers in the molecule. 2,3-diclorobutane has an intramolecular plane of symmetry in the eclipsed conformation, a centre in the anti conformation In Fischer projections: if two of the three identical groups are on the same side of the chain molecule on the two C’s (as in R,S-dichlorobutane) the two C’s have opposite configurations Molecules with more than one chiral centre (4) CH3 H Cl Cl H Cl H CH3 CH3 H Cl Cl H Cl H CH3 CH3 H Cl H Cl H Cl CH3 CH3 H Cl Cl H H Cl CH3 10.a 10.b 10.c 10.d 2,3,4-trichloropentane has 3 possibly asymmetric C’s. The possible stereoisomers should increase to (23): 4 diastereoisomers, each with its enantiomer as for 2,3,4-trichlorohexane. In 2,3,4-trichloropentane all three groups on the terminal C’s are identical and stereoisomers reduce to 4: In 10.a and 10.b (chiral enantiomers) atoms 2 and 4 are chiral and have the same configuration: S,S in a), and R,R in b). In both a) and b) atom 3, has identical groups and is non-chiral. Interchanging H and Cl of atom 3 and rotating by 180° leaves obviously the molecule unaltered. Molecules with more than one chiral centre (5) CH3 H Cl Cl H Cl H CH3 CH3 Cl H H Cl H Cl CH3 CH3 H Cl H Cl H Cl CH3 CH3 H Cl Cl H H Cl CH3 10.a 10.b 10.c 10.d In 10.c and 10.d atoms 2 and 4 are chiral with opposite configuration : S,R both in 10.c and 10.d. Atom 3 is pseudoasymmetric: C atoms on a molecular symmetry plane as in 10.c and 10.d are pseudoasymmetric: they are examples of prochiral centers. The two possible configurations of the pseudoasymmetric atom are labelled with the letters r (in d) and s (in c), R groups have higher priority than S. Any sp3 C of the kind CXYH2 is prochiral although not pseudoasymmetric. 10.c, 10.d and similar molecules are achiral. Atoms or groups related by a plane of symmetry on prochiral centres are enantiotopic ( pro-R e pro-S). They react in the same way with non-chiral species, but differently with chiral molecules. Atom C Pseudokiral CH3 H Cl Cl H Cl H CH3 A Atom C Nomor CH3 Cl H H Cl H Cl CH3 CH3 H Cl H Cl H Cl CH3 B CH3 H Cl Cl H H Cl CH3 C D 2 3 4 A S - S B R - R C S s R D S r R Pada C dan D atom C nomor 3 atom C pseudokiral (prioritas R > S) Atropoisomerism - biphenyls Biphenyls with bulky groups in positions a, b, c, d may be optically active and resolvable due to atropoisomerism Examples of resolvable biphenyls Atropoisomerism Paracyclophanes m = 2, n = 2 resolvable optically stable m = 3, n = 4 resolvable - racemizes at 160 °C m = 4, n = 4 non resolvable Examples of resolvable ansa-compounds