Shaun Lynn Grignard
advertisement

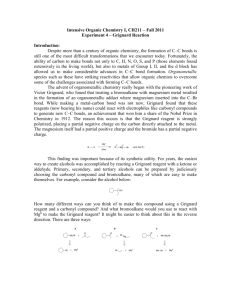
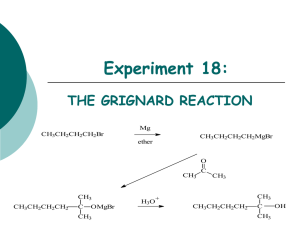
CARBON-CARBON BOND FORMATION: GRIGNARD ADDITION TO CARBON DIOXIDE SHAUN LYNN J9106574 Abstract A solution of bromo benzene in ether and magnesium were subjected to a nucleophilic addition reaction to produce the Grignard reagent phenyl magnesium bromide which was then reacted with crushed solid carbon dioxide diluted with sodium hydroxide and acidified with dilute hydrochloric acid to produce benzoic acid. The benzoic acid product was purified by recrystallisation then an infra red spectrum was obtained as well as a melting point for comparison to published literature. Introduction Born May 6th 1871, François Auguste Victor Grignard was awarded the Nobel Prize in Chemistry in 1912 for creating a new method of producing carbon – carbon bonds, using magnesium to couple ketones and alkyl halides. (The Nobel Foundation 1912, 2011) The Grignard reagent is prepared from a reaction of a halogenalkane with a suitable R-group (typically an alkyl or aryl group) and magnesium turnings with the use of dry diethyl ether as a solvent. Grignard reagents are versatile intermediates in the synthesis of organic functional groups and their reactions are extremely useful in organic synthesis, as they produce high yields of carbon – carbon bonds which are highly specific. Grignard reagents are strong bases as well as nucleophiles. Aim To prepare benzoic acid via the reaction of the Grignard reagent phenyl magnesium bromide with carbon dioxide. Hazards Ethyl ether – extremely flammable and highly volatile Solid carbon dioxide – may cause frostbite with prolonged contact Bromo benzene – flammable irritant. Hydrochloric acid – harmful, irritant may cause burns with prolonged contact Handling advice – This should be carried out in a fume cupboard. Method A three neck adapter was attached to a dry 250cm³ round bottomed flask. A water cooled condenser was dried, fitted with a CaCl2 drying tube and attached to the central neck of the 3 neck adapter. A solution of dry bromo benzene (10.5cm³) was prepared in dry ethyl ether (50cm³), poured into a dropping funnel and then attached to the 3 neck adapter. Magnesium turnings (2.5g) were weighed and added to the round bottomed flask. 10cm³ of the bromo benzene solution was then added to the round bottomed flask (just enough to cover the magnesium turnings). The round bottomed flask was then warmed over a water bath. After 10 minutes the solution turned a cloudy pale yellow. The remaining bromo benzene was then added drop wise. When all the solution had been added, the reaction vessel was left over the water bath for a further 20 minutes. Crushed solid carbon dioxide (20g) was weighed and added to the mixture, it was stirred until all the carbon dioxide disappeared. Dilute hydrochloric acid (2M) in excess was then added. The mixture was then poured into a separating funnel (250cm³) and lightly shaken until all the solid material dissolved. The liquid separated into two layers, an ethereal layer and an aqueous layer. The aqueous layer was discarded and dilute sodium hydroxide (2M) was added to the ethereal layer. The extracts were then collected in a conical flask and slowly acidified with dilute hydrochloric acid (2M) The precipitated benzoic acid was collected via filtration using a Buchner funnel. The sample was then transferred to a conical flask to be recrystallised, however and unknown organic solid contaminant was found in the bottom of the solution. The solution needed to be decantered to separate the two. The sample was then recrystallised again. Once cool, the sample was then filtered in a Buchner funnel and the purer benzoic acid product was transferred to a watch glass and left in a preheated oven (105°C) until dry. Discussion Mass of watchglass Mass of Watchglass & product Mass of product = 36.43g = 41.52 = 5.09g Melting point range = 121.9°C – 122.8°C The Dictionary of organic compounds, 6th edition, Chapman and Hall, London, Volume 3(& Volume 6), 1996 states that Pure benzoic acid has a melting point of 121.1°C – 122.35°C Which suggests the sample of benzoic acid is quite pure. Calculations Mr of C6H5Br = 157.0079 𝟏𝟎. 𝟓𝒈𝑷𝒉𝑩𝒓 𝒙 ( 𝟏 ) = 𝟎. 𝟎𝟔𝟔𝟖𝟕 𝒎𝒐𝒍𝒆𝒔 𝟏𝟓𝟕. 𝟎𝟎𝟕𝟗 𝟏 𝟐. 𝟓𝒈 𝑴𝒈 𝒙 (𝟐𝟒.𝟑𝟎𝟓 ) = 𝟎. 𝟏𝟎𝟐𝟗 𝒎𝒐𝒍𝒆𝒔 As bromobenzene was the limiting reagent in the production of phenylmagnesium bromide, 0.06687 moles of PhBr will produce 0.06687 moles of PhMgBr. Mr of PhMgBr = 181.3129 0.06687𝑚𝑜𝑙𝑒𝑠 𝑥 181.312 = 12.124𝑔 𝟏𝟐. 𝟏𝟐𝟒𝟑𝒈𝑷𝒉𝑴𝒈𝑩𝒓 𝒙 ( 𝟐𝟎𝒈 𝑪𝑶𝟐 𝒙 ( 𝟏 ) = 𝟎. 𝟎𝟔𝟔𝟖𝟕 𝒎𝒐𝒍𝒆𝒔 𝟏𝟖𝟏. 𝟑𝟏𝟐𝟗 𝟏 ) = 𝟎. 𝟒𝟓𝟒𝟒 𝒎𝒐𝒍𝒆𝒔 𝟒𝟒. 𝟎𝟎𝟗𝟓 For the overall reaction Bromobenzene is the limiting reagent. Moles are then converted to grams 0.06687𝑚𝑜𝑙𝑒𝑠 𝑥 157.0079 = 10.5𝑔 Theoretical yield = 10.5g Percentage yield = 5.09 10.50 𝑥100 = 48.476% The Mechanism of the Grignard reactions is as follows: R- +MgBr + O O C C O H3O+ R C O- +MgBr O R OH This reaction takes advantage of the high nucleophilicity of the phenyl portion of the Grignard reagent and the electrophilicity of the carbon atom of CO2. The Grignard reagent attacks the carbon of CO2 to form a carboxylate salt that is readily converted to benzoic acid by acidification with hydrochloric acid. The use of dry ice as the source of carbon dioxide helps regulate the reaction, because the extremely low temperature of this solid moderates the usual high exothermicity of Grignard additions. IR interpretation An O-H group is present in the molecule (identified by the region between 2500-3300 cm-1) Absorption at 1677.55 shows that a C=O is present. Absorption at 1288.67 cm-1 again shows the presence of an OH group. Absorption at 931.65 cm-1 could be due to the benzene ring or to the carbon-oxygen bond of the acid group. The spectrum was obtained using a Diamond ATR spectrometer. The spectrum obtained supports the statement that the sample is benzoic acid. WORKS CITED Clark, J. (2011, 04 04). Grignard Reagents. Retrieved 04 http://www.chemguide.co.uk/organicprops/haloalkanes/grignard.html 04, 2011, from chemguide: OrganicChemistry.org. (2011, 04 04). Grignard Reaction. Retrieved 04 04, 2011, from Organic Chemistry Portal: http://www.organic-chemistry.org/namedreactions/grignard-reaction.shtm Unknown. (2011, 04 01). Microsoft Word - GRIGNARD_INSTR.doc. Retrieved 04 01, 2011, from http://itech.pjc.edu/tgrow/2211L/grignard_instr.pdf Dictionary of organic compounds, 6th edition, Chapman and Hall, London, Volume 3(& Volume 6), 1996 McMurry, J. (2008). Organic Chemistry. J. McMurry, organic Chemistry . London: Thompson Brooks/Cole.