Chemistry 125: Lecture 67
April 12, 2010
Oxidizing/Reducing Alcohols
Grignard Reactions
Green Chemistry
This
For copyright
notice see final
page of this file
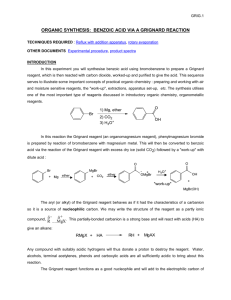
Alcohol Oxidation (sec. 16.14)
Stopping at Aldehyde (p. 805-6)
Pyridinium Chlorochromate (Cl-CrO2O-)
no water; no diol;
no overoxidation
(Loudon)
“In practice the chromium byproduct deposits with pyridine as
a sticky black tar, which can complicate matters.” (Wikipedia)
Vicinal Diol Cleavage by IO4- (sec. 16.14b)
Good Practice to Fill in Reagents
0:49-11:35
Alcohol (retro)Synthesis
(secs. 16.13, 16.15)
Hydride Reduction (sec. 16.13 p. 802)
“Versatility” of Grignard Reagent
(1 step from CH3OH)
O from CH OH)
nucleophilic(3 steps
3
carbon?
CH2from
O alkene
O
CH
O
O
C
C
or
Is there a
preferred order?
R-OH
PBr3
R-Br
Mg
R-MgBr
“Versatility” of Grignard Reagent
1) CH3MgBr
O
2) H+ / H2O
Cf. 2 t-Bu t-Bu-H
avoid steric +
hindrance
CH3
O
+
H MgBr
MgBr
OH
95%
H- reduction
1) t-BuMgBr
t-Bu
OH
2) H+ / H2O
OH
H
H
O
0%
H
O
no H
1) t-BuCH2MgBr
2) H+ / H2O
65%
CH2-t-Bu
OH
35%
H-CH2-t-Bu
H+
+ enolate ketone
:-(
4%
H-t-Bu
+ ketone
90%
from Roberts & Caserio (1965)
“Versatility” of Grignard Reagent
H
and steric
hindrance
Risk of Reduction
from Roberts & Caserio (1965)
Wittig Reaction (sec. 16.17)
Ph3P is good at taking up O
to form strong Ph3P=O bond.
Biological Oxidation
NAD+ , NADH revisited (sec. 16.18)
Pharmaceuticals generate < 0.2% of the chemical
industry’s product mass, but some 25% of its $ value,
and >50% of its chemical waste.
“Key green chemistry research areas - a perspective from pharmaceutical manufacturers”
Green Chemistry, 2007, 9, 411-420
Table 1 Reactions companies use now but would strongly prefer better reagents
Research Area
Number of roundtable companies voting
for this research area as a priority area
Amide formation avoiding poor atom economy reagents
6 votes
OH activation for nucleophilic substitution
5 votes
Reduction of amides without hydride reagents
4 votes
“Lithium aluminummethods
hydride,
having
molecular
weight
of 38 and
four
Oxidation/Epoxidation
without
theause
of chlorinated
solvents
4 votes
hydrides per molecule, has the highest hydride density and is frequently
Saferused,
and“…the
more
friendly Mitsunobu
reactions
3 votes
use ofit stoichiometric
high-valent
metals
evenenvironmentally
though
cogenerates
an inorganic
by-product
which
is
Os,
virtually been
eliminated
difficult (Mn,
toreaction
separate
fromhave
the product…slow
filtration
and product
loss
Friedel-Crafts
on Cr)
unactivated
systems
2 votes
throughfrom
occclusion
or adsorptionprocesses…”
are typical problems…”
pharmaceutical
Nitrations
2 votes
End of Lecture 67
April 12, 2010
Copyright © J. M. McBride 2010. Some rights reserved. Except for cited third-party materials, and those used by visiting
speakers, all content is licensed under a Creative Commons License (Attribution-NonCommercial-ShareAlike 3.0).
Use of this content constitutes your acceptance of the noted license and the terms and conditions of use.
Materials from Wikimedia Commons are denoted by the symbol
.
Third party materials may be subject to additional intellectual property notices, information, or restrictions.
The following attribution may be used when reusing material that is not identified as third-party content:
J. M. McBride, Chem 125. License: Creative Commons BY-NC-SA 3.0