Grignard Experiment
advertisement
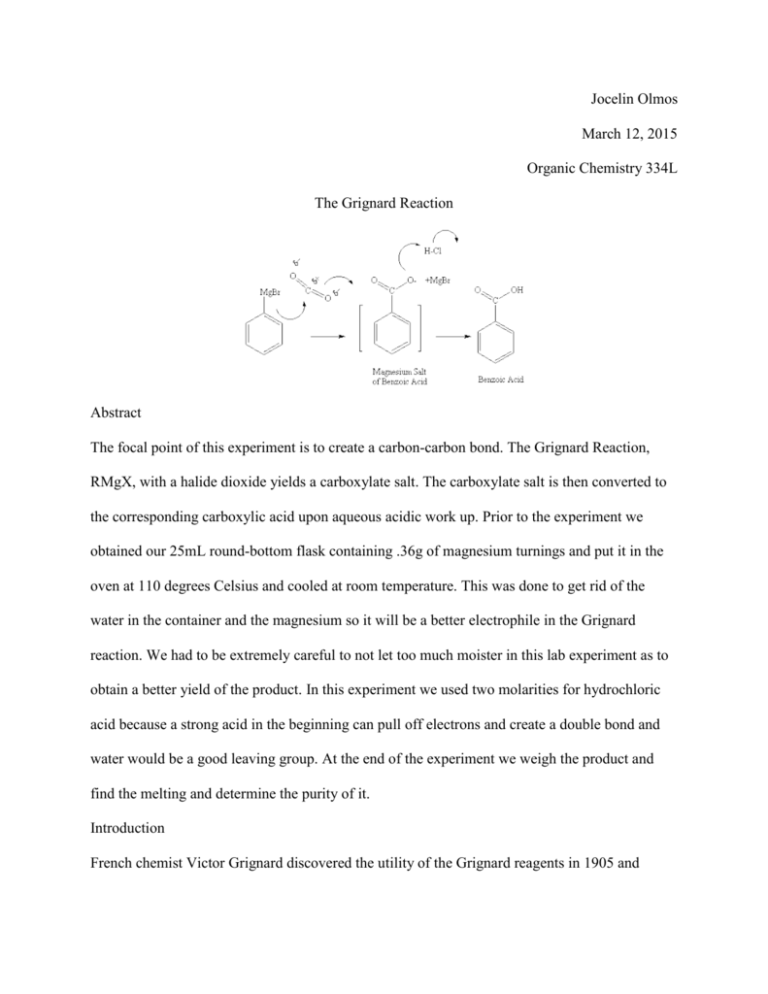
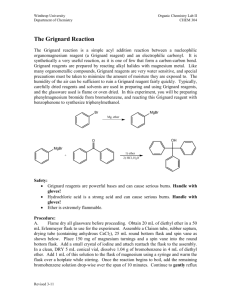
Jocelin Olmos March 12, 2015 Organic Chemistry 334L The Grignard Reaction Abstract The focal point of this experiment is to create a carbon-carbon bond. The Grignard Reaction, RMgX, with a halide dioxide yields a carboxylate salt. The carboxylate salt is then converted to the corresponding carboxylic acid upon aqueous acidic work up. Prior to the experiment we obtained our 25mL round-bottom flask containing .36g of magnesium turnings and put it in the oven at 110 degrees Celsius and cooled at room temperature. This was done to get rid of the water in the container and the magnesium so it will be a better electrophile in the Grignard reaction. We had to be extremely careful to not let too much moister in this lab experiment as to obtain a better yield of the product. In this experiment we used two molarities for hydrochloric acid because a strong acid in the beginning can pull off electrons and create a double bond and water would be a good leaving group. At the end of the experiment we weigh the product and find the melting and determine the purity of it. Introduction French chemist Victor Grignard discovered the utility of the Grignard reagents in 1905 and received a Nobel Prize in Chemistry in 1912. The Grignard reagent is the development of organometallic compounds of lithium and magnesium to synthesize alcohols. The reaction is made using a dry anhydrous ether solvent that is needed to solvate and stabilize the Grignard reagent as it forms. Grignard reagents can be made from primary, secondary, and tertiary halides, as well as from aryl and vinyl halides. The most reactive of the halides are iodide, then followed by bromides and chloride. Grignard reagents are strong nucleophiles and bases. The most useful reaction is the addition of the Grignard reagent to the carbonyl group. The Grignard reagent first adds to the carbonyl group to add a alkoxide ion. An addition of a dilute acid in a separate step protonates the alkoxide to give the alcohol. The significance of the Grignard reagent to organic chemistry is that it is one of the best methods to assemble a carbon skeleton with a strong base and nucleophile. Above is an example in the book regarding the Grignard reagent using a carbonyl. Overview The Grignard Experiment begins when we obtain the oven-dried apparatus from the instructor. We attach the round bottom flask with the water-cooled reflux condenser and a CaCl2 filled drying tube and we attach water lines and immediately replace the condenser and start cooling the water, we then add one crystal of iodine through the top of the condenser. We turn on the magnetic stirrer and it will stir the reaction vigorously. The agitated magnesium turnings provide sufficient impetus to initiate reaction. The disappearing of the iodine color, the cloudiness in the diethyl either and the gentle boiling of the solution indicate that the reaction has started. Once the reaction starts the diethyl ether should boil on its own. If the boiling stops, place the flask in a warm water bath and maintain a gentle reflux for a total reaction time of forty five to fifty minutes. Remove from the water bath and allow cooling. While it is cooling weigh approximately 3.0g of crushed dry ice into a 50mL beaker and slowly, but steadily pour the solution of phenylmagnesium bromide into this beaker. Stir reaction with glass stir rod until it sets into a gummy paste. Continue to stir until all the excess of carbon dioxide has evaporated. Add 7.5mL of 6 M of hydrochloric acid followed by 10mL of diethyl ether to the beaker. Add the acid very carefully. Keep checking that the pH is acidic. Transfer into a 60mL separatory funnel. Rinse the beaker with 1 or 2mL of the diethyl ether and add this rinse to the funnel. Shake the funnel vigorously and allow the layers to separate and set aside the aqueous lower layer. Extract the original diethyl ether upper layer with two 5mL portions of 5% sodium bicarbonate solution. Warm the combined extracts briefly in a sand bath to evaporate any excess diethyl ether. Acidify the mixture to a Congo Red paper end-point by drop wise addition of concentration hydrochloric acid with constant stirring. Isolate the precipitated benzoic acid by vacuum filtration and wash the crude product with a few mL of cold water while on the filter. Air dry the product until next laboratory session and weigh the dry product and calculate the experiment yield. Determine the melting point range of the benzoic acid and compare the result with the literature value. What went wrong with my experiment was in the beginning of the experiment when I was adding the 25mL round bottom flask to the cool condenser I did not properly attach them and the round bottom flask fell and broke scattering all the magnesium. Conclusion In conclusion the Grignard Experiment was created for a carbon-carbon bond. This experiment was created to see how the magnesium bromide can be a strong nucleophile and yield an alcohol.