Test Study Guide - ANSWER KEY - Liberty Union High School District
advertisement

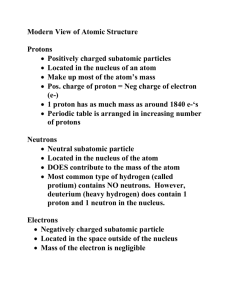
Name: _______________________________________________ Date: ______________________________ Period: _________ Unit 4 Test Study Guide Part 1: History of the Atom Sketch the atom models for the following scientists and summarize their experiments that led to their models. Democritus Greek who believed in atomos (matter made up of indivisible atoms) John Dalton British chemist who developed five postulates of atomic theory, thought they were indivisible spheres like Democritus J. J. Thomson Discovered negatively charged electron through cathode ray tube experiment Ernest Rutherford Niels Bohr Discovered nucleus, and most of atom was empty space Electrons traveled on circular, quantized orbits around nucleus Atomic Model Comparison Sketch each scientist’s model of the atom in the boxes given. Thomson Bohr Rutherford Democritus/Dalton Part 2: The Atom Complete the following table. Element Symbol Atomic # Atomic Mass # protons # neutrons # electrons Argon Ar 18 40 18 22 18 Cadmium Cd 48 112 48 64 48 Praseodymium Pr 59 140 59 81 59 Lead Pb 82 207 82 125 82 Tungsten W 74 184 74 110 74 Nuclear Symbol 40 Ar 18 112 Cd 48 140 Pr 59 207 Pb 82 184 W 74 Part 3: The Ion Complete the following table. Element Ion Symbol Atomic # Atomic Mass # protons # neutrons # electrons Cobalt Co2+ 27 59 27 32 25 Nuclear Symbol (include charge) 59 Co2+ 27 27 3+ Al 13 106 Pb2+ 46 133 2+ Cs 55 127 I 53 Aluminum Al3+ 13 27 13 14 10 Palladium Pb2+ 46 106 46 60 44 Cesium Cs2+ 55 133 55 78 53 Iodine (-1) I- 53 127 53 74 54 Atomic Mass # protons # neutrons # electrons Part 4: The Isotope Complete the following table. Element Hyphen Notation Atomic # Vanadium Vanadium-51 23 51 23 28 23 Vanadium Vanadium-52 23 52 23 29 23 Barium Barium-139 56 139 56 83 56 Lead Lead-209 82 209 82 127 82 Silver Silver-109 47 109 47 62 47 Nuclear Symbol 51 V 23 52 V 23 139 Ba 56 209 Pb 82 109 Ag 47 Part 5: Weighted Average Atomic Mass Solve the following problems + round your answer to the correct number of significant figures. 1. What is the average atomic mass of silicon given the following abundance information on the isotopes of silicon? Mass number Abundance Si-28 92.21 % Si-29 4.70 % Si-30 3.09 % 92.21 = 0.9221 100 0.9221(28) = 25.8188 4.70 = 0.0471 100 0.0471(29) = 1.3659 3.09 = 0.0309 100 0.0309(30) = 0.927 28.1117 28.1 amu 2. Calculate the atomic mass of potassium if the abundance atomic masses of the isotopes making up its naturally occurring samples are as given below. 93.12 = 0.9312 (38.964) = 36.283276 Isotope Relative abundance Atomic Mass 100 potassium-39 93.12 % 38.964 amu potassium-41 6.88 40.962 amu 6.88 = 0.0688 (40.962) = 2.8181856 100 39.101461 39.101 amu 3. What is the average atomic mass of hafnium given the following abundance information on its isotopes? Mass number Abundance Hf-176 5% Hf-177 19 % Hf-178 27 % Hf-179 14% Hf-180 35% 5/100 = 0.05(176) = 19/100 = 0.19(177) = 27/100 = 0.27(178) = 14/100 = 0.14(179) = 35/100 = 0.35(180) = 8.8 33.63 48.06 25.06 63 178.55 amu 180 amu 4. Neon has two isotopes. There are 800 atoms of Neon (with an average atomic mass of 20.3405 amu). 70% of the atoms are Neon-20 (19.8865 amu). What is the mass of the other Neon isotope? (Write it in hyphen notation and nuclear). 70% - 19.8865 ___% - ? Average atomic mass = 20.3405 amu 100% - 70% = 30% 70% - 19.8865 0.70 – 19.8865 30% - ? 0.30 - ? 0.70(19.8865) + 0.30(?) = 20.3405 13.92055 + 0.30(?) = 20.3405 -13.92055 -13.92055 0.30(?) = 6.41995 0.30 0.30 ? = 21.399833 The atomic mass of the 2nd isotope is 21.3998 amu.