HPLC/LC-MS Laboratory
advertisement

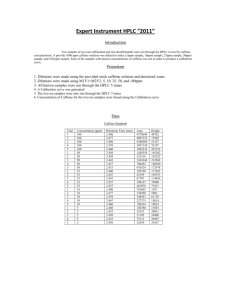
HPLC/LC-MS Laboratory High Pressure Liquid Chromatography and Liquid Chromatography Mass Spectrometry Purpose: The objective of this lab was to become familiar with the HPLC and LC-MS by running standard caffeine solutions and by running and analyzing the caffeine content in caffeinated and decaffeinated teas. Introduction: Liquid chromatography is a method used to separate the components of a compound that has been dissolved in a solvent. The sample is run through a liquid mobile phase and a liquid or solid stationary phase in a pressurized column. The LC-MS uses a mass spectrometer detector in order to separate the components by their mass to charge ratio in order to analyze what is in the sample. The HPLC can be used for quantitative analysis of a sample, showing the amount of substance in a sample. Day 1: Run standard samples of caffeine through the LC-MS for comparison to the samples and to gain an understanding of the instrument. Day 2: Run caffeine standards through the HPLC and run tea samples through HPLC and LC-MS. (Note: IC was also supposed to be included in this laboratory, but due to time constraints we were only able to run HPLC and LCMS) Method: Caffeine standards were made by dissolving caffeine in distilled water. Concentrations of standards were 50ppm, 100ppm, 200ppm, 300ppm, 400ppm, and 500ppm. Standards were run on HPLC and LCMS to create a calibration curve. Two tea samples were made (Bigelow English Teatime, caffeinated and decaffeinated) by boiling 150mL of distilled water for each tea bag, and letting the tea bag steep for four minutes with regular stirring. Tea samples were run through HPLC and LCMS for analysis of caffeine concentration. All data and spectra are located in supplementary folder. HPLC Data Concentration 50 ppm 200 ppm 300 ppm 400 ppm 500 ppm Caffeinated Tea Decaffeinated Tea Area 131119 2500 81616 199458 331786 31561624 46924160 Concentration vs. Area y = 497.85x + 4918.5 350000 300000 Area 250000 200000 150000 100000 50000 0 0 100 200 300 400 500 600 Concentration (ppm) Calculations: Caffeinated 𝑦 = 497.85𝑥 + 4918.5 31561624 = 497.85𝑥 + 4918.5 𝑥 = 63385.97𝑝𝑝𝑚 Decaffeinated: 𝑦 = 497.85𝑥 + 4918.5 46924160 = 497.85𝑥 + 4918.5 𝑥 = 94243.73𝑝𝑝𝑚 LCMS Data Concentration 50 ppm 100 ppm 200 ppm 300 ppm 400 ppm 500 ppm Caffeinated Tea Decaffeinated Tea Intensity, cps 8.2e6 7.6e6 6.6e6 6.3e6 6.3e6 6.1e6 1.3e6 2.4e6 Concentration vs. Intensity y = -4389x + 8E+06 9.00E+06 8.00E+06 Intensity (cps) 7.00E+06 6.00E+06 5.00E+06 4.00E+06 3.00E+06 2.00E+06 1.00E+06 0.00E+00 0 100 200 300 400 Concentration (ppm) 500 600 Calculations: Caffeinated 𝑦 = −4389𝑥 + 8 × 106 1.3 × 106 = −4389𝑥 + 8 × 106 𝑥 = 1526.54𝑝𝑝𝑚 Decaffeinated: 𝑦 = −4389𝑥 + 8 × 106 2.4 × 106 = −4389𝑥 + 8 × 106 𝑥 = 1275.92𝑝𝑝𝑚 Conclusion: In this lab, we were certainly able to learn how to use the HPLC and LCMS successfully. Both instruments were very easy to learn how to run, and they did not give us any problems during the experiment. The results, however, were certainly not what we expected. After using the calibration curves we created with the standards, we calculated the concentrations of the caffeine in the tea samples, but the calculations for the HPLC and for the LCMS were vastly different from each other. The data acquired from the LCMS is probably the closest to accurate, but the data and calculations we got from this experiment definitely show some error.