HPLC and LCMS
advertisement

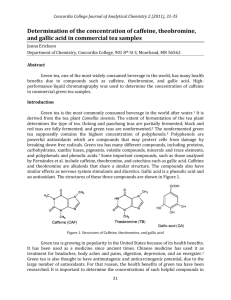
Liquid Chromatography – Mass Spectrometry and High Pressure Liquid Chromatography Introduction: Liquid chromatography uses a liquid (usually H2O) to push the solution containing the analyte onto the column. Depending on the polarity of the analyte relative to water determines the retention time of the analyte on the column. The more water soluble the analyte, the longer it is retained on the column. The converse is true as well, the less soluble in water the analyte is, the shorter it is retained on the column. Purpose: We will run standard samples of varying concentrations of caffeine on the LC-MS. We will determine the retention time based on the chromatograms. After we determine the retention time of caffeine, we will then run our unknown samples of Iced Tea. We will run regular Rutters Iced Tea as well as decaffeinated Iced Tea. We will then use the data we obtained from our standard samples to determine the presence of caffeine in both the regular and decaffeinated iced tea samples. We will be running standard samples on the HPLC. Our standards are aqueous solutions of caffeine in varying concentrations. Once we run the standards on the HPLC, we will determine retention time in order to determine if caffeine is present in regular Rutters Iced Tea as well as in decaffeinated Rutters Iced Tea. Procedure: 1. Make sure everything is turned on. Turn the nitrogen tank in the gas cylinder room on. The gauge on the left should read about 40 psi. Then open the nitrogen valve on the bench, it should also read approximately 40 psi. 2. Open ‘Analyst Software’ icon on the desktop. Then click on hardware configuration. Click LCMS then click Activate Profile. Click build acquisition batch. Fill in the set name (filename), select method caffeine 2011, click add set then click add sample. 3. Select the number of samples and then enter the numerical position of each vial. Then click submit (you have to click it twice). 4. Click view then go to sample queue. Click ready (located next to the T button) then click start sample. 5. To view the results, right click on the graph and select list data. Then open the Peak List tab and click view. Record any required data. 6. To shut down the LC-MS, click hardware config, then LCMS, then deactivate profile. 7. Then turn off the nitrogen gas, first the valve on the lab bench, then the tank in the cylinder storage room. Data: Concentration Standards Time (minutes) 10 ppm standard 1.7356 20 ppm standard 1.7055 30 ppm standard 1.6685 50 ppm standard 1.6884 100 ppm standard 1.6799 Area % Area 9.3910e5 counts 1.7165e6 counts 7.0834e6 counts 1.3719e6 counts 1.1563e6 counts 5.87% 73.83% 6.33% 10.87% 11.49% 8000000 y = -14719x + 3E+06 R² = 0.0406 7000000 6000000 5000000 4000000 3000000 2000000 1000000 0 0 20 40 60 80 100 120 HPLC: Caffeinated Tea Peak # 1 2 3 4 5 6 7 8 9 Retention Time 0.884 1.478 1.741 2.307 2.533 3.675 4.141 5.24 8.071 Area 219290 9360053 11996103 4761067 2670732 6255841 23709668 2025015 2859 The peak at 2.307 matches up with all the peaks that we got for our caffeine standards on the HPLC. From the intensity of our standard peaks compared to the intensity (area) of the caffeinated tea we were able to determine approximate concentration of caffeine in the sample. This was determined to be approximately 27 ppm. We weren’t able to run a decaffeinated tea sample because of time constraints. We were having issues getting the HPLC to run properly. Not really sure what happened, it was just not cooperative. Conclusions: With the LCMS we were able to determine that decaffeinated tea has very trace amounts of caffeine in it, <1 ppm.