HPLC, LC
advertisement

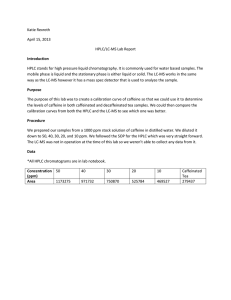
Experiment 1: HPLC, LC-MS, IC Introduction: HPLC stands for High Pressure (or Performance) Liquid Chromatography. Primarily used for separations of compound mixtures. This can be used for identification, quantity, and purity measurements. It uses a column separation technique, in which the analyte is separated by mobile and stationary phases under intense pressure. A detector provides retention times with a chromatogram of the samples eluted. LC-MS is Liquid Chromatography–Mass Spectrometry. This instrument utilizes a similar column separation technique employed in HPLC, but with mass analysis in the form of mass spectrometry. After separation, at the end of the column, solvent is removed and the sample is ionized. A mass detector then scans the molecules (by mass) and produces a spectrum identifying the compounds within the sample. IC stands for Ion Chromatography. Major use is the separation of ions and polar molecules based on charge. Typical example uses are water analysis and quality control analysis. A sample is placed into the column that contains a resin or gel matrix. The analyte is then detected by conductivity or UV/Vis absorbance. A graph is then created with characteristic peaks to specific ions in solution. Day One Objective: Use of the HPLC and LC-MS in the standardization of caffeine samples for later testing of tea samples. Equilibrate and run a standard on IC for later testing of water samples. Procedure: LC-MS 1. 2. 3. 4. 5. 6. 7. 8. Ensure everything is turned on. Turn on Nitrogen (40psi). Click Analyst Software icon on desktop. Click hardware configuration>LC-MS>Activate Profile. Click Build Acquisition Batch>method Caffeine10>add set> add sample> Select # of samples and vial positions>submit twice. Click View>Sample Queue Click Ready>Start Sample View results: Click Open Data File and find set name. Right Click on graph and click list data. Find peak list tap and click to view. 9. Click hardware configuration>LC-MS>Deactivate profile 10. Turn off Nitrogen in gas room. HPLC 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Check levels of methanol and water. Check pump lines A and B for air bubbles, prime lines if needed. Turn detector on. Select HPLC on desktop>Shimadzu>Methods>TSW.met Select Parameters>download>ok Inject when program says “waiting for trigger” Click analyze to show retention times and peak areas. Click Report icon>Area% Turn method to LOWFLOW.met and download. Turn detector off, and ensure methanol and water levels. 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Check Helium gas and set to 80psi Check bottles on instrument, refill if necessary. Open Chromeleon program on desktop Click File>Open>Change to control panel>MyPannels>Open ICS-90 Equilibrate for 20 to 30 mins Click File>New>Sequence>Next Set injection parameters>Next Click Batch Menu>Edit>Add. Inject sample. Click File>Open>Select Sequence> sample name. Name peaks by sheet in drawer. Close program when done, check fluid levels. Refill if necessary. IC Data: *Note: Chromatograms, and Peaks from IC are contained in Lab Notebook.* Standard solutions were diluted from 1000ppm to 50, 80, 100, 500ppm respectively (HPLC and LC-MS). Calibration Curve (HPLC) 14000000 12000000 A r e a 10000000 8000000 6000000 y = 28389x - 272711 R² = 0.989 4000000 2000000 0 0 100 200 300 400 500 600 Concentration (ppm) Standard solution was obtained from lab bench. Calibration Curve (IC) Peak Height (micro-S) 55 50 y = 5.6397x - 9.9185 R² = 0.4714 45 40 35 30 25 20 15 4 5 6 7 Retention Time Calculations: Dilution factors: 50ppm: 𝑉 = 50𝑝𝑝𝑚×100𝑚𝐿 1000𝑝𝑝𝑚 80ppm: V=8mL 100ppm: V=10mL V=5mL 8 9 500ppm: V=50mL Day Two Objective: Prepare tea samples. Repeat of day one procedures in running tea samples on HPLC and LC-MS. Run water samples on IC. Procedure: 1. Repeat Procedures from Day one. 2. Brew Teas and dilute for HPLC. Data: From HPLC Standards Calibration Curve: -Caffeinated English tea: Y=28389x-272711 For 1mL 𝑥 = x=concentration 255815+272711 28389 y=area x=18.62ppm For 2.5mL x=32ppm For 5.0mL x=35.7ppm -Caffeinated Organic Lab Tea: For 1mL x=12.5ppm For 2.5mL x=27.1ppm For 5.0mL x=62.7ppm -Decaffeinated English Tea: Undiluted sample =24.1ppm Conclusion: HPLC Yielded better results overall in the three instruments used. Sample size was an issue, standards over 550ppm plateau and became useless. Caffeinated tea samples yielded good results, caffeine is definitely present. Decaffeinated yielded some caffeine results(expected). This concentration fell between diluted sample sizes, so basically in a whole serving would constitute a very small fraction of the beverage. LC-MS yielded poor results in the standards, all had extreme background noise except for the 1000ppm sample. A calibration curve was impossible. However the sample data yielded great results showing caffeine present, but without a calibration curve, the concentration remains unknown. IC was a fickle instrument, requiring a long preparation time and a long sample run (approximately 16 minutes). We were able to obtain a standard and one sample, however on day two the IC developed issues and no other samples were ran. The cambell hall sample yielded decent results with 3 peaks present.