Structure and Transformation of Matter Review Sheet
advertisement
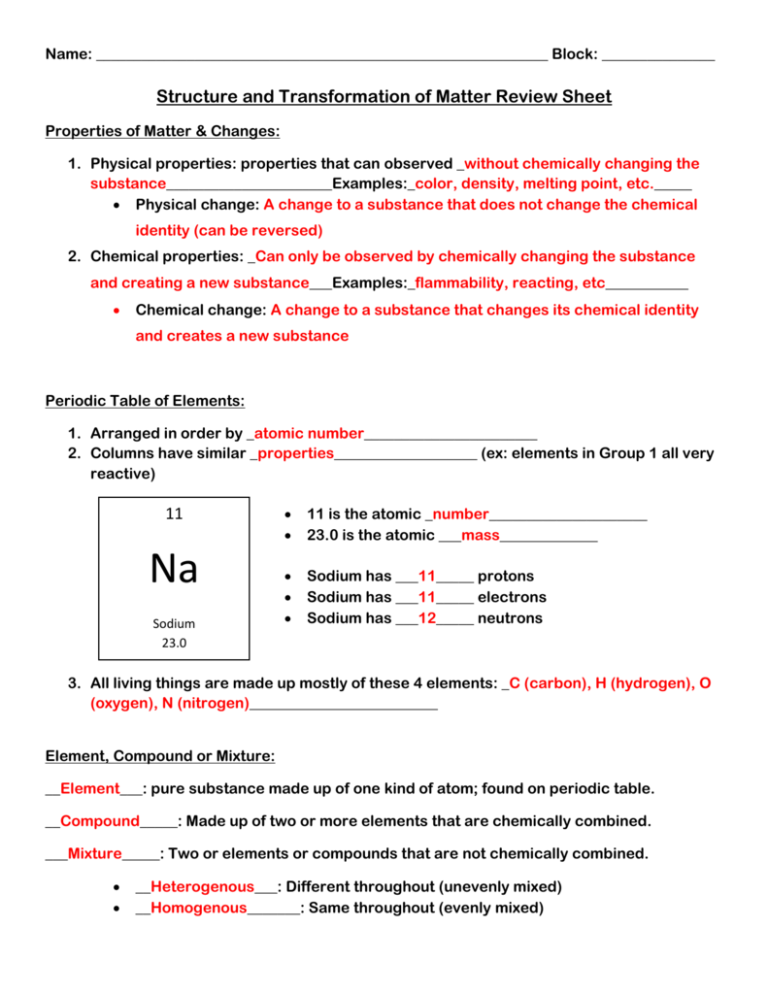
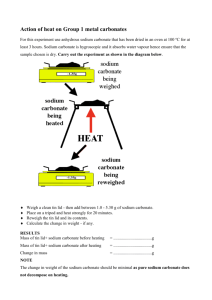
Name: ____________________________________________________________ Block: _______________ Structure and Transformation of Matter Review Sheet Properties of Matter & Changes: 1. Physical properties: properties that can observed _without chemically changing the substance______________________Examples:_color, density, melting point, etc._____ Physical change: A change to a substance that does not change the chemical identity (can be reversed) 2. Chemical properties: _Can only be observed by chemically changing the substance and creating a new substance___Examples:_flammability, reacting, etc___________ Chemical change: A change to a substance that changes its chemical identity and creates a new substance Periodic Table of Elements: 1. Arranged in order by _atomic number_______________________ 2. Columns have similar _properties___________________ (ex: elements in Group 1 all very reactive) 11 Na Sodium 23.0 11 is the atomic _number_____________________ 23.0 is the atomic ___mass_____________ Sodium has ___11_____ protons Sodium has ___11_____ electrons Sodium has ___12_____ neutrons 3. All living things are made up mostly of these 4 elements: _C (carbon), H (hydrogen), O (oxygen), N (nitrogen)_________________________ Element, Compound or Mixture: __Element___: pure substance made up of one kind of atom; found on periodic table. __Compound_____: Made up of two or more elements that are chemically combined. ___Mixture_____: Two or elements or compounds that are not chemically combined. __Heterogenous___: Different throughout (unevenly mixed) __Homogenous_______: Same throughout (evenly mixed) Name: ____________________________________________________________ Block: _______________ Write E, C or M if it is an element, compound or mixture: H2O - C Trail Mix - M Salt water - M He - E Copper - E Hydrogen - E NaOH - C Oil & water - M Kool Aid - M HCl - C Carbon - E Hg - E Sugar water - M Aluminum - E NaCl - C Chemical Reactions: Chemical reactions involve two main kinds of change: 1. Formation of a __new________ __substance________________ Examples: a. Gas bubbles when sodium carbonate and hydrochloric acid are combined b. Solid formed when two liquids (copper sulfate solution and sodium carbonate solution) are combined. This is called a _precipitate___________________ 2. Changes in __energy__________________ Example: a. Heating/burning sugar over a candle flame b. __Endothermic________ heat is taken in (like the sugar/candle) c. __Exothermic_________ heat is given off (like the fireworks) The rate (speed) of a chemical reaction can be affected by which factors (and do they increase or decrease the rate of the reaction): Heat (temp), surface area, concentration – (increasing these speeds up reaction); Catalysts (can increase or slow down a reaction) Chemical Equations: What is a chemical equation? _A short way to show what happens in a chemical reaction____________________________________________________________________ _Reactants____________ : are the substances that react in a chemical reaction _Products____________: are the substance produced in a chemical reaction C + O2 → CO2 Which numbers are coefficients? Big #’s in front (1’s in this case) Which numbers are subscripts? Little #’s below (2’s in this case) Is this equation balanced? yes Balance and rewrite the equation. CH4 + 2O2 → CO2 + H2O Which numbers are coefficients? 1’s and 2 Which numbers are subscripts? 4, 2, 2, 2 Is this equation balanced? No Balance and rewrite the equation. CH4 + 2O2 → CO2 + 2H2O