Unit 4 SPM - Northwest ISD Moodle
advertisement
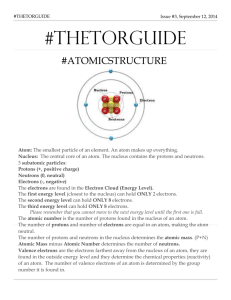
UNIT 4.1-Atomic Structure and Properties Student Progress Monitoring (SPM) 1. Atomic Structure- 8.5 A We will describe the masses, electrical charges and locations of protons, neutrons and electrons. Vocab: Electron Electrical charge Subatomic particle Proton Electron cloud Nucleus Neutron Atomic mass number Neutral Atom Atomic number Positive/negative Questions: A. Describe the locations and electrical charges of a proton, electron, and neutron. B. Explain the similarities and differences of the masses of the subatomic particles. C. How can you determine the number of protons, neutrons and electrons in an atom using the Periodic Table? D. What is the electrical charge of an atom’s nucleus and why? What is the electrical charge of an atom’s electron cloud and why? 2. Protons and Valence Electrons 8.5B • We will identify that protons are an atom’s identity and that valence electrons determine reactivity and other chemical properties. Vocab: Valence Electron Reactivity Proton Questions: A. B. C. D. How is the identity of an atom determined? Define valence electron and explain the importance of them. How many electrons can be held in the 1st 3 energy levels (shells) Define reactivity and what determines an element’s reactivity. Energy level(shell) Chemical property UNIT 4.1-Atomic Structure and Properties Student Progress Monitoring (SPM) 3. Atomic Structure- 8.5 A We will describe the masses, electrical charges and locations of protons, neutrons and electrons. Vocab: Electron Electrical charge Subatomic particle Proton Electron cloud Nucleus Neutron Atomic mass number Neutral Atom Atomic number Positive/negative Questions: E. Describe the locations and electrical charges of a proton, electron, and neutron. F. Explain the similarities and differences of the masses of the subatomic particles. G. How can you determine the number of protons, neutrons and electrons in an atom using the Periodic Table? H. What is the electrical charge of an atom’s nucleus and why? What is the electrical charge of an atom’s electron cloud and why? 4. Protons and Valence Electrons 8.5B • We will identify that protons are an atom’s identity and that valence electrons determine reactivity and other chemical properties. Vocab: Valence Electron Reactivity Proton Questions: E. F. G. H. How is the identity of an atom determined? Define valence electron and explain the importance of them. How many electrons can be held in the 1st 3 energy levels (shells) Define reactivity and what determines an element’s reactivity. Energy level(shell) Chemical property