Prep Chemistry Course Objectives 2013-2014
advertisement

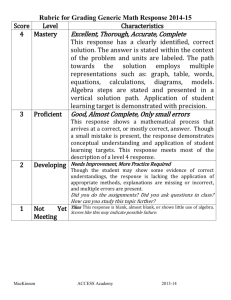
2013-2014 Course Syllabus Subject: Prep Chemistry Teacher: Ms. Tomar Location: RM N233 Email: stomar@houstonisd.org Welcome to Chemistry! Please read the following guidelines carefully and ask if you have any questions. I. General Guidelines for Our Class: 1. RESPECT. Treat everyone like you want to be treated. Property (including laboratory equipment) should also be treated respectfully. You are financially responsible for lab and classroom equipment. 2. RESPONSIBILITY. You choose your behavior, and you earn your grade. Remember every action/choice has a consequence. 3. ATTENDANCE. We have a lot to learn about science in a short amount of time. So, you are expected to be seated, working on your warm-up, and ready to participate when the bell rings. Tardiness to class is disruptive to your teacher and classmates. Should you be late to class, you will find the door locked. Go and get a tardy pass from the deans’ office before entering the classroom. Absences: Every day of class is important. It is important that you attend and participate. * When you return to class: a. Check the absent tray for your make-up work. You will have one day for every day you were absent to complete and turn in your make-up work. b. Talk to your lab partner to discuss lab work, assignments, notes, etc. c. Talk to me for lab make-up. You are responsible for preparing for the lab by completing the prelab assignment, and labs should be made up within one week of returning. If you do not arrive on time or if you are not prepared, you may forfeit your lab make-up opportunity. b. Check with me to set up due dates for any missing assignments. c. Turn in any assignments that were due the day you were absent. * If you are absent only on the day of a test or quiz, you are responsible for taking the test or quiz on the day you return. If you have been absent several days prior to a quiz or exam, speak with me to schedule the new test or quiz date 4. PREPARATION. Completing homework and bringing your materials to class allows us to get the most out of the time and energy we spend in the classroom. Please don't expect to go to your locker. Here is a list of materials you are responsible for bringing to class: scientific calculator with log button. 2” three-ring binder with notebook paper and dividers. Label the dividers as follows: warm-ups, notes, work in progress, and graded work pens (red and blue or black ink) and pencils 5. APPROPRIATE LANGUAGE and COMMENTS. We want to maintain an environment for learning and expressing ideas and opinions appropriately. This includes having a positive attitude. 6. ACADEMIC HONESTY. Cheating will not be tolerated and will be handled in accordance with school policy. I believe that working with other students, in class and out, provides many benefits, and I realize that studying together is an excellent learning tool when used properly. Be sure, when working with others, that you really are helping each other to understand the material. For lab reports with partners, your data section should be identical, and you may work together to analyze the laboratory results, but your write-up should be your own. In the event that lab reports or other assignments are turned in with identical or nearly identical work, all participants will receive zeros. 7. ASK QUESTIONS. Whether you need clarification or just want to learn more, you should feel free to ask questions. If you need additional support outside of class, I am available to help you during tutorials on Mondays and Fridays during lunch, or by appointment. 8. SAFETY. In the classroom and in the lab, your safety is paramount. Please refer to the Laboratory Safety Agreement. II. Grades Your grades will be based on the level of understanding you demonstrate on assignments and assessments. Homework is assigned and is used to deepen your understanding of the concepts. 70% (Major Grades): tests, major labs, and major projects 30% (Minor Grades): quizzes, classwork, homework, and class participation III. Test Corrections If you obtain a test grade below 70, you can come to tutorials and attempt test corrections. The guidelines for test corrections are: Corrections must be done on a separate piece of paper stapled to the front of the original test For each missed question for which you want to recover points, you need to do the following: 1. Write out the question number you got wrong. 2. Write out the question you got wrong. 3. Write the correct answer. 4. Show all work for math-based problems. 5. Write a sentence (or two) explaining WHY it’s the correct answer. To EXPLAIN is not to state. It is to give a reason. 6. Explain why your answer was wrong. 7. Have your parent/guardian sign the corrections. 8. Corrections must be completed and turned in within one week of the day when grades were posted in the online gradebook. Example of test corrections done correctly: Question #3 You are given a compound with the formula MCl2 in which M is a metal. You are told that the metal has 26 electrons. What is the identity of the metal? The correct answer is Ni, because somewhere along the way the metal lost 2 electrons (b/c it has a +2 charge). Originally it must have had 28 electrons. My answer was Fe because I know that Fe has 26 protons, so I figured it had 26 electrons as well. Examples of test corrections done incorrectly: 1. I read the problem wrong. 2. I wrote down the wrong answer. 3. I don’t know. 4. I mixed my numbers up. *I believe all of these statements are true. However, for credit to be awarded, learning of the concepts must be evident in the test corrections. Recovery Points: You can recover up to 50% of the points that you lost on the test, provided you meet the aforementioned requirements for test corrections, including correct explanations and proper reflection. IV. Late Work For minor grade assignments, late work will not be accepted and will be entered in the gradebook as a zero, except in cases of documented family or medical emergency. It’s a strict policy because you cannot afford to get behind in this class. For major grade assignments that are not tests, you will lose 11 points for every day the assignment is late. I look forward to a successful school year together! Parent/Guardian Contact Information: Student Name: _____________________________________________ Class Period: _____ Please have your parent or guardian complete this section or please complete it about your PARENT. Parent/Guardian: ____________________________________ Address: ____________________________________ ____________________________________ Phone: ____________________________________ Alternate Phone: ____________________________________ Parent Email: ____________________________________ Which of the above is the best method to contact you? ____________ What time of day would you prefer to be contacted? _____________ I have read and fully understand what is expected from me during this course. Parent Signature Thank you for your input! Ms. Shivangi Tomar stomar@houstonisd.org Student Signature Date Prep Chemistry Course Objectives 2013-2014 (subject to modifications) First Semester First Six Weeks I. Nature of Science a. Lab safety & equipment b. Scientific methods c. Scientific measurement d. Graphs and data analysis II. Matter a. Characteristics and classification of matter b. Physical and chemical properties c. Physical and chemical changes Second Six Weeks I. Periodic Table a. Historical development b. Properties of chemical families c. Periodic trends II. Atomic Structure a. Historical development b. Electromagnetic spectrum and its mathematical relationships c. Calculations using Planck’s constant and the speed of light d. Electron configurations III. Nuclear Chemistry a. Types of radiations b. Balancing of nuclear equations c. Fusion and fission reactions Third Six Weeks I. Bonding a. Naming of compounds using IUPAC nomenclature rules b. Writing of chemical formulas c. Electron dots diagrams d. Metallic bonds e. Predictions of molecular structures using VSEPR theory Prep Chemistry Course Objectives 2013-2014 (subject to modifications) Second Semester Fourth Six Weeks I. Chemical Reactions a. Mole concept b. Use of the mole concept to calculate chemical quantities—stoichiometry c. Percent composition d. Empirical and molecular formulas e. Balancing of chemical reactions—Law of Conservation of Matter f. Calculations of limiting reactants and percentage yields Fifth Six Weeks I. Gases a. Kinetic Molecular Theory b. Gas laws c. Stoichiometric calculations II. Thermochemistry a. Energy and its forms b. Energy changes calculations—Law of Conservation of Energy c. Specific heat concept d. Calorimetry Sixth Six Weeks I. Solutions a. b. c. d. e. f. g. h. i. Properties of water Solubility rules Types of solutions Molarity concept Factors that affect solubility and rates of dissolution Acids and bases definitions pH calculations Degrees of dissociation Differentiating types of chemical reactions