Electron Note Outline
advertisement

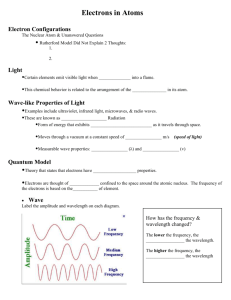
Electron Arrangement In the early 1900’s, scientists thought light behaved solely as a ______________. This belief changed when light was later discovered to have ____________ - _________________ ______________________________. Today, light is thought to have both ________________ and ______________________ properties. THE WAVE DESCRIPTION OF LIGHT ________________________________________________ is a form of energy that exhibits wavelike behavior as it travels through __________________. Electromagnetic radiation travels at a speed of __________________ in air, which is 3.0 x 108 meters/second. Electromagnetic Radiation has the measureable wave-like properties of _______________________ and _____________________________. Wavelength – the ________________________ between corresponding points on adjacent ________________. Frequency – the ____________________________ of waves that pass a given point in a specific amount of time, usually __________________________. c = λν c - ______________ λ - _____________ ν - ___________ In summary, as the wavelength of light decreases, the frequency increases and vice versa. This is an ___________________ relationship. Electromagnetic spectrum – all the electromagnetic radiation arranged according to _________________________. THE PARTICLE DESCRIPTION OF LIGHT ___________________________________________ - the emission of electrons by metals when light is shined on them. These observations led scientists to believe that light had particle-like properties. They proposed that the wavelike radiation emitted from the object was in small, specific amounts called __________________________. _______________________________ is a minimum quantity of energy that can be gained or lost by an atom. A ____________________ is a particle of electromagnetic radiation having ______________ mass and carrying a ________________ of energy. Energy of a photon is ________________________ proportional to the frequency of radiation. E = hν E - ________________________ h = Planck’s constant, 6.626 x 10-34 J*sec THE HYDROGEN-ATOM LINE-EMISSION SPECTRUM When atoms in the gaseous state are heated, their _____________________________ increases. The atoms return to their original energy state, and they give off the added energy. The state of lowest energy is called the _________________________________________. The state in which it has a higher potential energy than the ground state is called an __________________________. When a narrow beam of emitted light was shined through a prism, it was separated into a series of specific frequencies and wavelengths of visible light. The bands of light were part of what is known as hydrogen’s ____________________ ________________________ _____________________________. Wave theory views the waves as forms of energy. The Particle Theory views the particles as pieces of matter. Modern view uses a composite of these two theories to explain light. Einstein’s Theory of Relativity (E = mc2) combines both matter and energy into one formula containing the speed of light. BOHR MODEL OF HYDROGEN 1. The single electron of the _______________atom can circle the nucleus only in allowed paths called _____________. 2. When an electron is in these orbits, the atom has a _____________ ______________ _________________. 3. The _______________ ____________ __________________ occurs when the electron is in the orbit closest to the nucleus. 4. The total energy of the electron ___________________ as it moves into orbits _______________ from the nucleus. An electron moves to a higher energy orbit when excited. The Heisenberg Uncertainty Principle and the Schrodinger Wave Equation laid the foundation for modern quantum theory. The Heisenberg Uncertainty Principle states that it is _________________________ to determine simultaneously both the ____________________ and ____________________ of an electron or any other particle. Schrodinger used the dual wave-particle nature of electrons to develop an equation that treated electrons in atoms as _____________________. Quantum Theory describes mathematically the ___________________ properties of ______________ and other very small particles. QUANTUM NUMBERS AND ATOMIC ORBITALS _____________________________________ are the numbers that specify the properties of atomic orbitals and properties of their electrons. They indicate the region occupied by a given orbital in terms of the following: 1st quantum number indicates the ______________ ______________ __________________or the ____________ from the nucleus. 2nd quantum number indicates the orbital ___________________________. 3rd quantum number indicates the orbital _________________________ with respect to the x, y and z axis. *** The first three quantum numbers result from solutions to Schrodinger’s equations 1. Principal quantum number – symbolized by ______________, indicates the __________________surrounding the nucleus. These energy levels are sometimes referred to as _____________________________. Values of n are ____________________________________. n= 1 is the ____________________ that is ____________________ to the ______________________________. As n ___________________________, the distance of the energy levels from the nucleus _________________. 2n2 = the number of _____________ that can fit in each level (n is the energy level) 2. Angular Momentum quantum number (l) – indicates the _____________________ of the orbital. Within each energy level _____________________ with different shapes occupy different __________________________. The regions are called _______________________ or _________________________. The first four angular momentum quantum numbers are designated in ascending order by the letters _______________________. _________ is the lowest in energy, __________ is the next level, then d and f. S sublevel is a __________________ shape. P sublevel is a ___________________ shape. D sublevel is a ________________ __________________ shape. F is too complex to describe. Principal Quantum Number (energy level) Types of Orbitals 1 1s 2 2s, 2p 3 3s, 3p, 3d 4 4s, 4p, 4d, 4f 3. Magnetic quantum number (m) – indicates the ______________ of an orbital about the __________________. S sublevel has ________ orientation around the nucleus. P sublevel has ________ orientations around the nucleus. D sublevel has ________ orientations around the nucleus. F sublevel has ________ orientations around the nucleus. 4. Spin quantum number – has only two possible values, which indicate two possible states of an electron in an orbital. +1/2 = _______________ spin -1/2 = ______________________ spin First Level: s orbital = holds no more than ___________ electrons Second Level: S orbital = (1) holds no more than _________ electrons P orbital = (3) holds no more than _________ electrons TOTAL: 8 electrons Third Level: S orbital = (1) holds no more than ____ electrons P orbital = (3) holds no more than ____ electrons D orbital= (5) holds no more than ____ electrons TOTAL: 18 electrons Fourth Level: S orbital = (1) holds no more than ____ electrons P orbital = (3) holds no more than ____ electrons D orbital= (5) holds no more than ____ electrons F orbital= (7) holds no more than ____ electrons TOTAL: 32 electrons RULES FOR WRITING ELECTRON CONFIGURATIONS Electron configuration is the ______________________ of the electrons within _____________. Atoms of different elements have _________________ numbers of ______________________. Electrons in atoms tend to assume arrangements with the _________________________ possible energies. Pauli Exclusion Principle: no two ________________ in the same atom can have the same set of four ______________ numbers. Hund’s Rule: orbitals of ____________________ are each occupied by one electron before any orbital is occupied by a second electron, and all ________________________ in singly occupied __________________ must have the same spin. Aufbau Principle: an electron occupies the _________________________________ that can receive it. *Two Exceptions to the Aufbau Principle Cr 1s12s22p63s23p64s13d5 Cu 1s12s22p63s23p64s13d10 Aufbau Diagram: (draw) ELECTRON CONFIGURATIONS Orbital notation: an unoccupied orbital is represented by a ______________. An orbital with one electron is represented by a __________. An orbital containing two electrons is represented as _____________. The lines are labeled with the principle quantum number and sublevel letter H _____ He_____ Li ___ ____ Na ____ ____ _____ _____ _____ _____ Electron –configuration notation: the number of electrons in a sublevel is represented by adding ________________ to the sublevel designation H – 1s1 He - ________ Li- __________ Na - ___________ Noble Gas notation: a shortcut electron configuration that substitutes a portion of the electron configuration with a ______________ ____________ (group 18 – pick the noble gas closest in atomic number, without going over). Noble gases are used because they all have a filled outer shell of electrons, called an _________________. Examples: Br Try: Sulfur: Barium: [Ar] 4s23d104p5 Electron –dot notation: show only ________________ electrons, which are those in the __________________ principal quantum number. Examples: 1) 1s22s22p63s23p4 has _______ valence electrons. What element is this? _____________ Electron dot? ______ 2) 1s22s22p63s23p64s23d5 has _______ valence electrons. What element is this? ________ Electron dot? ______ 3) 1s22s22p63s23p64s23d104p5 has ____ valence electrons. What element is this? _______ Electron dot? _______ Electron Dot Samples: 1 valence electron: 5 valence electrons: 2 valence electrons: 6 valence electrons: 3 valence electrons: 7 valence electrons: 4 valence electrons: 8 valence electrons: PRACTICE PROBLEMS: 1. 2. 3. 4. 5. The electron configuration of boron is: 1s22s22p1 a. How many electrons are present in a boron atom? b. What is the atomic number for boron? c. Write the orbital notation for boron: d. How many valence electrons does boron have? e. Write the electron dot symbol for boron: The electron configuration of fluorine is 1s22s22p5 a. How many electrons are present in a fluorine atom? b. What is the atomic number for fluorine? c. Write the orbital notation for fluorine: d. How many valence electrons does fluorine have? e. Write the electron dot symbol for fluorine: Lithium has the electron configuration of _________________________________________________. Calcium has the electron configuration of _________________________________________________. Write the electron configuration for Ca using noble gas notation ______________________________. QUANTUM NUMBER PRACTICE: 1. 2. 3. 4. 5. 6. 7. 8. How many sublevels are possible in the 2nd energy level? How many sublevels are possible in the 3rd energy level? How many sublevels would you expect to exist in the 5th energy level? How many orbitals are in a p sublevel? How many orbitals are in a d sublevel? How many electrons can fit in an f sublevel? How many orbitals are found in the entire 3rd energy level? How many electrons can fit in the entire 3rd energy level?