Chapter Two Review: Sections 2.1-2.5
advertisement

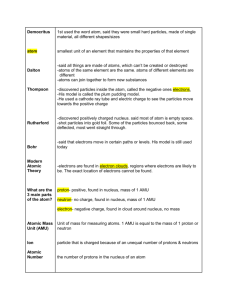
Honors Chemistry Name: _______________________________________ Date: _______________ Mods: __________ Chapter Two Review: Sections 2.1-2.5 1. Match the following scientist with their experiment/contribution to atomic theory: a. John Dalton _______1. First to believe there was a piece of matter b. Ernest Rutherford which would be indivisible c. Robert Millikan _______2. Developed the understanding radioactivity d. JJ Thompson _______3. Cathode ray tube experiment e. Democritus _______4. Atomic Theory which includes 3 Basic Laws f. _______5. Gold Foil Experiment Marie Curie _______6. Oil Drop Experiment ________6. Credited with discovering the electron from his experiment results and the charge to mass ratio of an electron ________7. Ancient Greek philosopher whose idea about matter was lost for over 2000 years ________8. Had evidence that supported a model of the atom which has a small, dense, positively charged center (nucleus). ________9. Incorrectly believed, and wrote in his theory, that the atom was indivisible ________10. Credited with calculating the charge of a single electron ________11. The first to propose atomic theory based on scientific evidence ________12. Hypothesized that the model of the atom was like “Plum Pudding” in that the majority of the atom was a sphere of positive charge with small negative particles spread evenly throughout it (like raisins in pudding). This resulted in an atom that had an even mass and charge distribution throughout it. ________13. His model of the atom was the first to propose that the electrons were separate from the positive charge of the atom (electrons outside of the nucleus) and that the majority of the atom was empty space ________14. Died from radiation poisoning 2. What is the name of each of the following groups? a. Group 1A: _________________________________________________________ b. Group 2A: _________________________________________________________ c. Group 6A/16: _________________________________________________________ d. Group 7A/17: _________________________________________________________ e. Group 8A/18: _________________________________________________________ 3. Identify the element based on its group/period assignment. a. The period 2 alkaline earth metal: _____________________________________ b. The period 5 Noble gas: _____________________________________ c. The period 6 alkaline earth metal: _____________________________________ d. The metalloid in group 16: _____________________________________ e. The only nonmetal in group 14: _____________________________________ 4. Use your periodic table to fill in the missing information in the chart below. Isotope of Element Atomic Mass Atomic Number 184 74 # protons 35 # neutrons # electrons 45 197 118 85 Rb 7 7 Copper-63 87 138 78 5. What is the average atomic mass of Germanium off of the periodic table? ____________________________. Taking this into consideration, which is more abundant, 72 Ge or 73Ge, assuming that these are its only two isotopes? Explain. 6. In nature, 20.3% of Boron exists as Boron-10 and the remainder is Boron-11. What is the average atomic mass of Boron (assume the isotopic masses are whole numbers)? 7. Nitrogen is made up of two isotopes, N-14 and N-15. The isotopic masses are 14.003074 amu and 15.000108 amu, respectively. Given nitrogen's average atomic weight is 14.007 amu, what is the percent abundance of each isotope?