Chapter 3 Jeopardy Chem Part 1 Atomic Theory and
advertisement
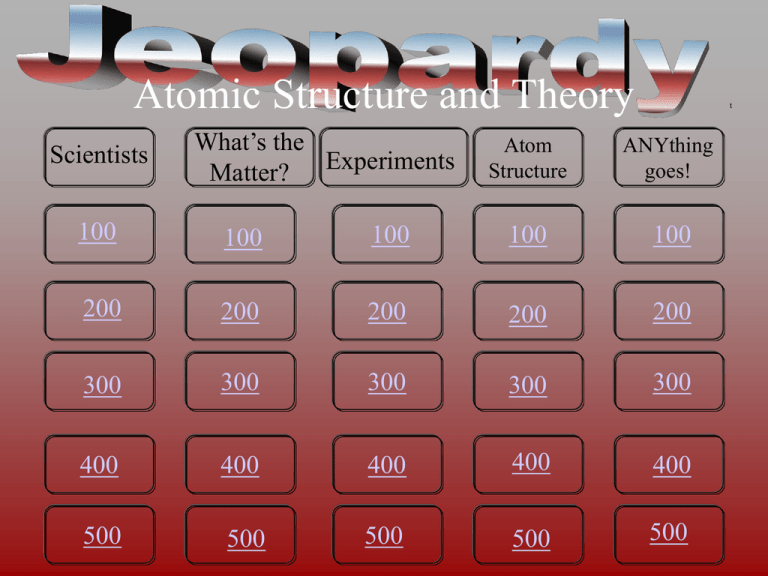
Atomic Structure and Theory Scientists What’s the Matter? Experiments t Atom Structure ANYthing goes! 100 100 100 100 100 200 200 200 200 200 300 300 300 300 300 400 400 400 400 400 500 500 500 500 500 I-100 Thought of the atom as “plum-pudding” . Answer I-200 Thought of the atom as a “planetary” model . Answer I-300 This scientist first discovered the nucleus. . Answer I-400 Thought of the “billiard ball” model for the atom . Answer I-500 Came up with the Electron Cloud model of the atom we use today. . Answer II-100 First modern scientist that said that matter is composed of indestructible and indivisible atoms . Answer II-200 This subatomic particle carries a negative charge . Answer II-300 When you have the same element, it is guaranteed that you have the same number of (these) subatomic particles. . Answer II-400 This scientist came up with the periodic table. . Answer II-500 The name of an atom that takes in or gives up electrons. . Answer III-100 The Gold foil experiment showed this about the volume of an atom. . Answer III-200 The charge carried by alpha particles and the charge carried by the nucleus . Answer III-300 These particles make up most of the mass of the atom and are found here. . Answer III-400 The subatomic particle that was discovered by J.J. Thompson in the late 1800's. . Answer III-500 The reason element tubes give off different colored spectral lines . Answer IV-100 This element has 32 electrons and 73 neutrons . Answer IV-200 This column of gases does not like to bond with other elements b/c they have a full valence shell. . Answer IV-300 Atomic mass minus the atomic number equals the number of this subatomic particle in an atom. . Answer IV-400 all atoms of the same element have the same…; give name and particle name . Answer IV-500 The name of the outermost shell of electrons that determines atom bonding. . Answer V-100 This element has 3 electrons and 4 neutrons . Answer V-200 The first electron shell holds this many electrons in the Bohr model. . Answer V-300 The net charge of an atom . Answer V-400 Opposite charges do this. . Answer V-500 Like charges do this. . Answer I-100 A • Who is JJ Thomson? Game board I-200 A • Who is Bohr? Game board I-300 A • Who is Rutherford? Game board I-400 A • Who is Dalton? Game board I-500 A • Who is Schrodinger? Game board II-100 A • What is Dalton’s Atomic Theory? Game board II-200 A What is an electron? Game board II-300 A • What are Protons? Game board II-400 A • Who is Mendeleev? Game board II-500 A • What is an ion? Game board III-100 A • What is mostly empty space? Game board III-200 A • What is positive? Game board III-300 A • What are neutrons and protons; the nucleus? Game board III-400 A • What is an electron? Game board III-500 A • What is energy makes the electron jump to a higher energy level and when it falls it gives off light. Game board IV-100 A • What is Germanium? Game board IV-200 A • What are the Noble Gases? Game board IV-300 A • What is number of neutrons? Game board IV-400 A • atomic number; protons Game board IV-500 A • What is valence shell? Game board V-100 A • What is Lithium? Game board V-200 A • the one with the decimal! Game board V-300 A • What is zero? Game board V-400 A • What is attract? Game board V-500 A • What is repel? Game board