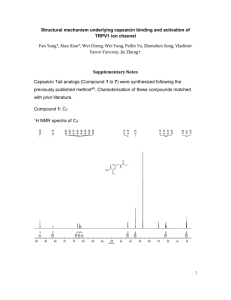
301 supporting information
advertisement

Synthesis and photovoltaic performance of N-dioctylmethyl-2,7-carba-zole-alt-5,7-bis(thiophen-2-yl)-2,3 -biphenyl-thieno[3,4-b]pyrazine copoly- meric derivatives appending various donor units in phenyl moieties † † Jianmin Li , Hua Tan , Fanyuan Meng, Wenhong Peng, Yafei Wang, LIU Yu & Weiguo Zhu* Key Lab of Environment-Friendly Chemistry and Application in Ministry of Education, College of Chemistry, Xiangtan University, Xiangtan 411105, China Corresponding author (email: zhuwg18@126.com) †Contributed equally to this work Received July 28, 2014; accepted August 14, 2014 1 Experimental 1.1 Materials Compounds 3, M1, M2, M4, M5, and M6 were synthesized according to the previously described procedures [2426]. Tetrahydrofuran (THF) and toluene were dried over sodium/benzophenone and freshly distilled prior to use. Other reagents and solvents were commercial grade and used as received without further purification. All reactions were performed under nitrogen atmosphere. 1.2 Measurement and characterization All synthesized compounds were characterized by nuclear magnetic resonance spectra (NMR). The NMR spectra were recorded on a Bruker AV-400 (American) using deuterated chloroform (CDCl3) as solvent. The average molecular weight and polydispersity index (PDI) of the copolymers were determined with a Waters (American) 1515 gel permeation chromatograph (GPC) using THF as an eluent and polystyrene as a standard. Thermogravimetric analyses (TGA) were performed on a Netzsch TG 209 analyzer (Germany) under nitrogen atmosphere at a heating rate of 20 °C min1. Surface morphologies were recorded by atomic force microscopy (AFM) on a Veeco, DI multi-mode NS-3D apparatus (American) in trapping mode under normal air conditions at room temperature (RT). UV-vis absorption spectra were measured on a PerkinElmer Lambda 25 spectrometer (American). Cyclic voltammetry (CV) measurements were conducted using a EG&G Princeton Applied Research Model 273 potentiostat/galvanostat (China) equipped with electrochemical analysis system software and a standard three-electrode configuration under an argon atmosphere at RT. Platinum rod, platinum wire, and an Ag/Ag+ electrode were respectively used as the working electrode, counter electrode and reference electrode, in a 0.1 mol L1 tetrabutylammonium hexafluorophosphate (Bu4NPF6) acetonitrile solution. Polymeric thin films on platinum rods were prepared by dipping the electrode into a 0.2–1.0 wt% chloroform solution and drying them under a house-nitrogen steam before measurement. 1.3 Polymer solar cell fabrication and test The photovoltaic cells were made with a traditional sandwich structure using the following steps. The indium-tin oxide (ITO)-coated glass substrates were respectively cleaned by a series of ultrasonic treatments in acetone, deionized water, and 2-propanol for 10 min. The substrates were then dried under a stream of nitrogen and subjected to the treatment of Ar/O2 plasma for 5 min. A filtered aqueous solution of poly(3,4-ethylenedioxy-thiophene)- poly(styrenesulfonate) (PEDOT:PSS; Bayer AG, American) was spin-cast onto the ITO surface at 2000 rpm for 30 s and then baked at 150°C for 30 min to form a PEDOT:PSS thin film with a thickness of 30 nm. A blend of copolymer and PC61BM (1:3 w/w, 20 mg mL1 for polymer) dissolved in chlorobenzene was filtered through a 0.45 um poly(tetrafluoroethylene) filter and spin-cast onto the PEDOT:PSS layer as the active layer at 3000 rpm for 30 s. The resulting substrates were dried under N 2 at RT and then annealed at 150 °C for 15 min in a nitrogen-filled glove box. The devices were obtained after a 10 nm lithium fluoride and a 0.5 nm aluminum were thermally deposited onto active layer as cathode under a pressure of 6×104 Pa. The active area was 9 mm2 for each cell. The thicknesses of the spun- cast films were recorded by a profilometer (Alpha-Step 200; Tencor Instruments, American). Device characterization was carried out under AM 1.5G irradiation with an intensity of 100 mW cm2 (Oriel 91160, 300 W) calibrated by a NREL-certified standard silicon cell. Current density-voltage (J-V) characteristics were measured with a computer-controlled Keithley 2602 source measurement unit (American) in the dark. PCEs were detected under a monochromatic illumination (Oriel Cornerstone 260 1/4 m monochromator equipped with Oriel 70613NS QTH lamp, American). The incident light was calibrated by a monocrystalline silicon diode. All device characterizations were performed under an ambient atmosphere at RT. 1.4 Synthesis of 1,2-bis(4-(5-octylthiophen-2-yl)phenyl) -ethane-1,2-dione (3) Tributyl(5-octylthiophen-2-yl)stannane (2) (1.50 g, 3.08 mmol), 1,2-bis(4-bromophenyl)-ethane-1,2-dione (0.52 g, 1.4 mmol), and PdCl2(dppf)2CH2Cl2 (0.0564 g, 0.08 mmol) were added into toluene (30 mL). The resultant mixture was stirred under nitrogen atmosphere and heated to 120 °C for 48 h. After being cooled to RT, the reaction mixture was extracted with dichloromethane (DCM). The organic layer was collected and dried over magnesium sulfate. The solvent was removed by rotary evaporation and the residue was purified by silica column chromatography (DCM/petroleum ether (PE), 1/3, v/v) to give Compound 3 as a yellow solid with a yield of 72.8%. 1H NMR (400 MHz, CDCl3, ppm): 7.97 (d, J=8.2 Hz, 4H), 7.68 (d, J=8.2 Hz, 4H); 7.40 (d, J=4.0 Hz, 2H), 6.80 (d, J=4.0 Hz, 2H), 2.83 (t, J=6.4 Hz, 4H), 1.70 (m, 4H), 1.28 (m, 20H), 0.88 (t, J=8.0 Hz, 6H). 1.5 Synthesis of Compound 5 To a mixture of Compound 4 (2.48 g, 4.8 mmol), 1,2-bis(4-bromophenyl)ethane-1,2-dione (0.80 g, 2.17 mmol), and Pd(PPh3)4 (0.14 g, 0.12 mmol) was added a mixing solution of THF (42 mL) and aqueous potassium carbonate (2 mol/L, 14 mL). The mixture was vigorously stirred at 75 °C for 24 h under a nitrogen atmosphere. After being cooled to RT, the reaction mixture was extracted with DCM. The organic layer was collected and dried over magnesium sulfate. The solvent was removed by rotary evaporation and the residue was purified by silica column chromatography (DCM/PE, 1/2, v/v) to give Compound 5 as a yellow liquid with a yield of 67.2%. 1H NMR (400 MHz, CDCl3, ppm): 8.11 (d, J=8.0 Hz, 4H), 7.82 (d, J=8.0 Hz, 4H), 7.78 (s, 2H), 7.75 (d, J=6.3 Hz,2H), 7.43 (t, J=6.0 Hz, 4H), 7.36 (d, J=5.2 Hz, 6H), 2.02 (t, J=6.4 Hz, 8H), 1.04 (m, 40H), 0.81 (m, 8H), 0.66 (t, J=8.0 Hz, 12H). 1.6 Synthesis of Compound 7 This compound was synthesized according to the synthetic procedure of Compound 5 and purified by silica column chromatography (DCM/PE 1/3, v/v). A yellow solid was obtained with a yield of 56.2%. 1HNMR (400 MHz, CDCl3, ppm): 8.38 (s, 2H), 8.16–8.01 (br, 6H), 7.89 (d, J=8.0 Hz, 4H), 7.78 (d, J=8.1 Hz, 2H), 7.50 (t, J=4.8 Hz, 4H), 7.44 (d, J=8.0 Hz, 2H), 7.28 (d, J=6.4 Hz, 2H), 4.35 (t, J=8.0 Hz, 4H), 1.91–1.88 (m, 4H), 1.40–1.25 (br, 20H), 0.86 (t, J=7.8 Hz, 6H). 1.7 Synthesis of Compound 10 This compound was synthesized according to the synthetic procedure of Compound 5 and purified by silica column chromatography (DCM/PE, 1/3, v/v). A red liquid was obtained with a yield of 60.1%. 1HNMR (400 MHz, CDCl3, ppm): 8.01 (d, J=8.1 Hz, 4H), 7.68 (d, J=8.1 Hz, 4H), 7.45 (d, J=8.4 Hz, 4H), 7.09 (d, J=8.5 Hz, 8H), 6.97 (d, J=8.4 Hz, 4H), 6.85 (d, J=8.5 Hz, 8H), 3.95 (t, J=6.8 Hz, 8H), 1.79–1.76 (m, 8H), 1.46(m, 8H), 1.32–1.29 (br, 32H), 0.89 (t, J=8.0 Hz, 12H). 1.8 Synthesis of Compound M2 5,5"-Dibromo-3',4'-dinitro-2,2':5',2"-terthiophene (1.488 g, 3.0 mmol), iron powder (2.18 g, 39 mmol), and acetic acid (60 mL) were mixed and heated under stirring at 60 °C for 30 min. The mixture was cooled to RT and filtered. The filtrate was mixed with benzil (0.48 g, 4.0 mmol) in a 100 mL flask and was stirred at 60 °C for 5 h under nitrogen atmosphere. After the mixture was cooled to RT, water was added. The precipitate was formed and filtered off, then washed with water and CH3OH. The collected precipitate was dried and purified by silica column chromatography (DCM/PE, 1/2, v/v) to give Compound M2 as a black-blue solid with a yield of 61.4%. 1H NMR (CDCl3, 400 MHz, ppm): 7.57 (d, J=7.1 Hz, 4H), 7.41–7.32 (m, 8H), 7.06 (d, J=3.9 Hz, 2H). 1.9 Synthesis of Compound M3 This compound was synthesized according to a synthetic procedure of Compound M2 and purified by silica column chromatography (DCM/PE, 1/4, V/V). A black solid was obtained with a yield of 43.2%. 1H NMR (CDCl3, 400 MHz, ppm): 7.62–7.56 (m, 8H), 7.32 (d, J = 3.8 Hz, 2H), 7.21 (d, J = 3.4 Hz, 2H), 7.06 (d, J = 3.8 Hz, 2H), 6.77 (d, J = 2.7 Hz, 2H), 2.84 (t, J = 6.0 Hz, 4H), 1.72–1.69 (m, 4H), 1.39–1.26 (br, 20H), 0.88 (t, J = 8.4 Hz, 6H). 1.10 Synthesis of Compound M4 This compound was synthesized according to a synthetic procedure of Compound M2 and purified by silica column chromatography (DCM/PE, 1/6, V/V). Black-green solid was obtained with a yield of 47.8%. 1H NMR (CDCl3, 400 MHz, ppm): 7.80–7.71 (br, 12H), 7.66 (d, J = 9.6 Hz, 4H), 7.35 (d, J = 5.4 Hz, 8H), 7.08 (d, J = 3.8 Hz, 2H), 2.02 (t, J = 6.4 Hz, 8H), 1.26–1.16 (br, 40H), 0.83–0.78 (m, 8H), 0.68 (t, J =8.2 Hz, 12H). 1.11 Synthesis of Compound M5 This compound was synthesized according to a synthetic procedure of Compound M2 and purified by silica column chromatography (DCM/PE, 1/2, V/V). A black solid was obtained with a yield of 45.5%. 1H NMR (CDCl3, 400 MHz, ppm): 8.41(s, 2H), 8.17 (d, J = 7.6 Hz, 2H), 7.79– 7.72 (br, 10H), 7.50–7.41 (br, 6H), 7.35 (d, J = 3.4 Hz, 2H), 7.24 (d, J = 7.4 Hz, 2H), 7.08 (d, J = 3.5 Hz, 2H), 4.34 (t, J = 4.8 Hz, 4H), 1.91–1.88 (m, 4H), 1.40–1.34 (br, 20H), 0.87 (t, J = 8.0 Hz, 6H). 1.12 Synthesis of Compound M6 This compound was synthesized according to the synthetic procedure of Compound M2 and purified by silica column chromatography (DCM/PE, 1/2, V/V). A black-red solid was obtained with a yield of 35.7%. 1H NMR (CDCl3, 400 MHz, ppm): 7.67 (d, J = 7.8 Hz,4H), 7.57 (d, J = 7.9 Hz, 4H), 7.47 (d, J = 8.2 Hz, 4H), 7.32 (d, J = 3.7 Hz, 4H), 7.08 (d, J = 8.6 Hz, 8H), 6.99 (d, J = 8.0 Hz, 4H), 6.84 (d, J = 8.4 Hz, 8H), 3.95 (t, J = 4.8 Hz, 8H), 1.79–1.74 (m, 8H), 1.45–1.29 (br, 40H), 0.89 (t, J = 6.0 Hz, 12H). 1.13 General procedures of Suzuki polycondensation, taking PCz-3ThPz-Ph for an example Monomers M1 (131 mg, 0.2 mmol), M2 (122 mg, 0.2 mmol), and dry toluene (10 mL) were added into a 25 mL double-neck round-bottom flask. The container was purged with N2 flow for 30 min to remove oxygen and Pd(PPh3)4 (5 mg) was added into it. The mixture was heated to 115 °C for 10 min and tetraethyl ammonium hydroxide (0.9 mL, 20% by weight in H2O) was then injected. The resultant mixture was heated to reflux under stirring and nitrogen atmosphere for 72 h. Phenylboric acid solution (50 mg in 0.5 mL toluene) was subsequently injected and the mixture was continued to reflux for another 6 h. Finally, bromo- benzene (0.5 mL) was injected and the mixture was allowed to reflux for 6 h. After being cooled to RT, the mixture was slowly poured into methanol (200 mL) and a lot of precipitate was formed. The precipitate was collected and extracted respectively with methanol, hexane, acetone, and chloroform in a Soxhlet apparatus for 12 h each. The chloroform solution was collected and distilled to remove the great mass of chloroform. The residual solution was dropped into methanol (200 mL) to yield precipitate. The precipitate was filtered and dried under vacuum overnight to give PCz-3ThPz as a green solid with a yield of 28.7%. 1H NMR (CDCl3, 400 MHz, ppm): 8.19–8.01 (br, 2H), 7.93-7.54 (br, 12H), 7.50–7.32 (br, 8H), 4.78–4.55 (br, 1H), 2.53–2.26 (br, 2H), 2.13–1.88 (br, 2H), 1.42–1.17 (br, 24H), 0.89–0.60 (br, 6H). PCz-3ThPz-PhTh: green solid, yield: 36.8%. 1H NMR (CDCl3, 400 MHz, ppm): 8.17–7.92 (br, 2H), 7.70–7.58 (br, 10H), 7.22 (br, 4H), 6.78 (br, 4H), 4.76– 4.52 (br, 1H), 2.95–2.65 (br, 4H), 2.51–2.24 (br, 2H), 2.12–1.92 (br, 2H), 1.85–1.64 (br, 4H), 1.42–1.14 (br, 44H), 0.99–0.51 (br, 12H). PCz-3ThPz-PhFl: green solid, yield: 55.8%. 1H NMR (CDCl3, 400 MHz, ppm): 8.23–8.04 (br, 2H), 7.90–7.67 (br, 22H), 7.55–7.41 (br, 2H), 7.41–7.30 (br, 4H), 7.28–7.07 (br, 2H), 4.79–4.54 (br, 1H), 2.54–2.26 (br, 2H), 2.15–1.78 (br, 10H), 1.41–1.07 (br, 72H), 0.86–0.54 (br, 18H). PCz-3ThPz-PhCz: green solid, yield: 52.9%. 1H NMR (CDCl3, 400 MHz, ppm): 8.53–8.24 (br, 4H), 8.24–8.00 (br, 4H), 7.98–7.54 (br, 14H), 7.54–7.31 (br, 8H), 7.31–7.04 (br, 2H), 4.40–4.02 (br, 5H), 2.54–2.23 (br, 2H), 2.11–1.63 (br, 6H), 1.26–1.08 (br, 44H), 0.90–0.51 (br, 12H). PCz-3ThPz-PhTpa: green solid, yield: 71.8%. 1H NMR (CDCl3, 400 MHz, ppm): 8.21–7.97 (br, 2H), 7.89–7.72 (br, 8H), 7.58–7.47 (br, 12H), 7.18–6.92 (br, 12H), 6.91–6.72 (br, 8H), 4.77–4.52 (br, 1H), 4.03–3.81 (br, 8H), 2.54–2.26 (br, 2H), 2.11–1.88 (br, 2H), 1.77– 1.75 (br, 8H), 1.45– 1.12 (br, 64H), 0.95–0.62 (br, 18H).