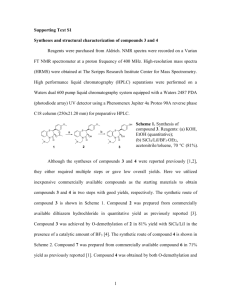
Supplementary Notes
advertisement
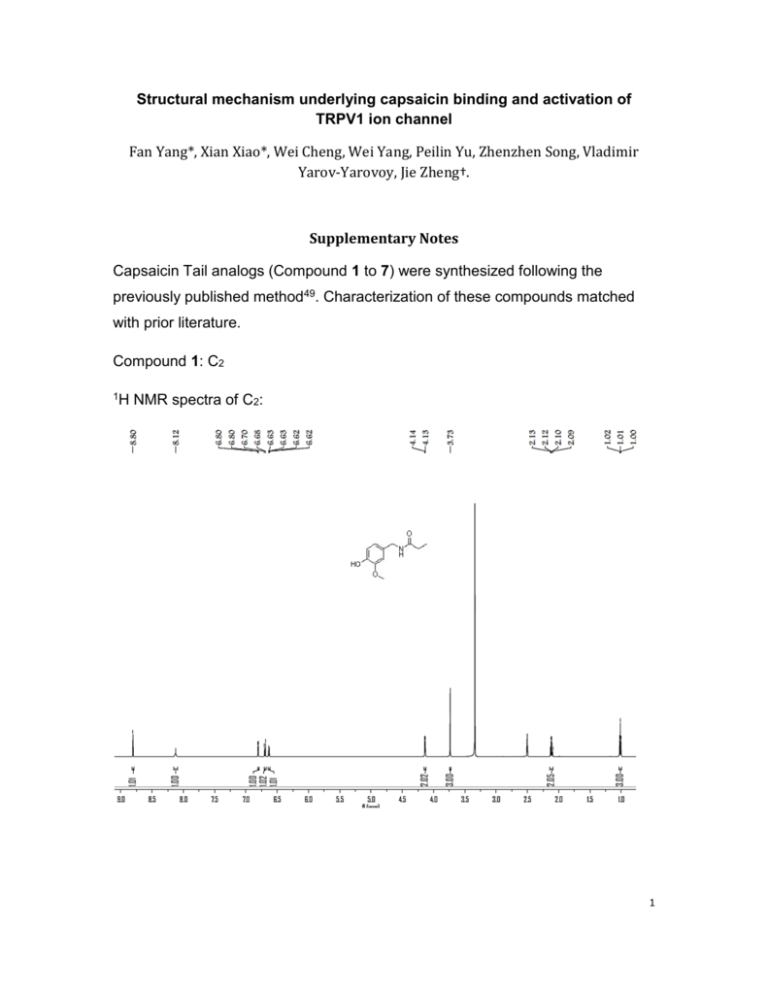
Structural mechanism underlying capsaicin binding and activation of TRPV1 ion channel Fan Yang*, Xian Xiao*, Wei Cheng, Wei Yang, Peilin Yu, Zhenzhen Song, Vladimir Yarov-Yarovoy, Jie Zheng†. Supplementary Notes Capsaicin Tail analogs (Compound 1 to 7) were synthesized following the previously published method49. Characterization of these compounds matched with prior literature. Compound 1: C2 1H NMR spectra of C2: 1 Compound 2: C3 1H NMR spectra of C3: 2 Compound 3: C4 1H NMR spectra of C4: 3 Compound 4: C5 1H NMR spectra of Analog C5: 4 Compound 5: C6 1H NMR spectra of Analog C6: 5 Compound 6: C7 1H NMR spectra of Analog C7: 6 Compound 7: C11 1H NMR spectra of Analog C11: 7 Compound 8: Compound C Capsaicin Neck analog (Compound C) was synthesized as previously reported50 (Reaxys database: Rx-ID 1827424). All synthesis reactions were carried out by introducing an atmosphere of argon. Column chromatography was performed on silica gel (300-400 mesh). The purity of each compound was determined by 1H NMR and 13C NMR recorded using CDCl3 as the solvent in a NMR spectrometer (Bruker) at 400MHz and 100 MHz. respectively. Synthesis pathway: A small amount of 4-Hydroxy-3-methoxy Benzylamine Hydrochloride (Intermediate 1) (0.95 g, 5 mmol) was dissolved in 40mL of CH2Cl2, Et3N (1.0 g, 10 mmol) was then added. Subsequently, An appropriate Acyl Chloride (Intermediate 2)(85%, 1.0 g, 4.5 mmol)in CH2Cl2 (15 mL) at room temperature (22ºC),was slowly added dropwise, using an injection pump within 20 hours. Once the Acyl Chlorides has been added, chemical reaction was allowed to proceed at room temperature for 24 hours. Then water was used for wash. The aqueous layer was extracted with CH2Cl2 twice. The combined organic layers were washed with brine, and then dried with anhydrous Na2SO4. Solvent was filtered and concentrated under reduced pressure to get crude products. Capsaicin was purified by column chromatography. The prepared Capsaicin (1.0g, 3.3 mmol) in toluene (20 mL) was added Lawesson’s Reagent (1.1 g, 2.6 mmol) and interacted at 80ºC for two hours. Then water and brine were used for washed in sequence, then dried with 8 anhydrous Na2SO4. Solvent was filtered and concentrated under reduced pressure to get crude products. Purification by column chromatography obtained the desired Compound 8 (Compound C). Characterization of these compounds matched with prior literature. H NMR (400 MHz, CDCl3) δ 7.25 (d, J = 5.9 Hz, 1H), 6.85 (ddd, J = 11.7, 9.8, 4.9 Hz, 1 3H), 5.41 – 5.25 (m, 2H), 4.74 (d, J = 5.0 Hz, 2H), 3.88 (s, 3H), 2.69 – 2.62 (m, 2H), 2.21 (dq, J = 13.2, 6.6 Hz, 1H), 2.04 – 1.95 (m, 2H), 1.79 (dt, J = 15.4, 7.7 Hz, 2H), 1.44 – 1.34 (m, 2H), 0.94 (d, J = 6.7 Hz, 6H). 9 C NMR (101 MHz, CDCl3) δ 205.26 (s), 146.81 (s), 145.66 (s), 138.21 (s), 128.12 (s), 13 126.38 (s), 121.49 (s), 114.63 (s), 111.09 (s), 77.34 (s), 77.02 (s), 76.71 (s), 56.00 (s), 50.36 (s), 47.13 (s), 32.18 (s), 30.96 (s), 28.87 (d, J = 3.1 Hz), 22.64 (s). 10 Compound 9: Compound D Capsaicin Head analog (Compound D) was synthesized as previously reported51 (Reaxys database: Rx-ID 311196 and 9064049) Synthesis pathway: 4-hydroxy-3-methoxy benzaldehyde (Intermediate 1) (4.6 g, 30 mmol) and anhydrous K2CO3 (4.8 g, 35 mmol) was mixed with acetone (80 mL) in a 250 mL round-bottomed flask. The mixture was oil-bathed at 45ºC for one hour. Then 6.5 g Ch3I (, 45 mmol) was slowly added dropwise, and the reaction was allowed to proceed overnight. Acetone was removed by speedVac concentrator, 5% HCl (60 mL) was then added. Ethyl acetate (60 mL) was used to extract for three times. The combined organic layers were washed with water and brine subsequently and then dried by anhydrous Na2SO4. Filtered and concentrated to generate Intermediate 2 (5.0 g). Intermediate 2 (5.0 g, 30 mmol) was dissolved in 95% ethanol. HONH2·HCl (2.7 g, 39 mmol) and NaHCO3 (3.4 g, 40 mmol) was added sequentially and heated for one hour .The reaction was followed by TLC. After cooling process, brine (70 mL) was added, and ethyl acetate (60 mL) was used to extract for three times. The combined organic layer was washed by water and brine subsequently, dried by anhydrous Na2SO4. Filtered and concentrated to generate Intermediate 3 (5.3 g). 11 LiAlH4 (0.6 g, 15 mmol) was added to tetrahydrofuran (THF, 25 mL). Intermediate 3 solution in THF was slowly added dropwise, in an ice bath for 30 min. Reaction was allowed to proceed overnight. After the reaction mixture was cooled to room temperature, it was diluted with diethyl ether (30 mL) and further cooled to 0ºC. Water (0.6 mL), 15% NaOH solution (0.6 mL), water (2 mL) and anhydrous Mg2SO4 was added sequentially, and then stirred for half an hour at room temperature before filtration. After solvent was evaporated, diethyl ether (20 mL) and HCl (0.2 M) was added. pH of the water phase was adjusted by Na 2CO3 to be alkaline. Then the water layer was extracted twice with CH2Cl2 (30 mL)..The combined organic layers were washed with water and brine subsequently, dried by anhydrous Na2SO4. Filtered and concentrated to generate Intermediate 4 (0.8 g). Intermediate 4 (0.5 g, 3.0 mmol) and NEt3 (0.3 g, 3.0 mmol) was dissolved in CH2Cl2 (40 mL). Intermediate 5 (0.5 g, 2.5 mmol, 85%) was added dropwise, at room temperature. Reaction was allowed to proceed at room temperature for three hours. After reaction was complete, solution was washed by water and brine, and then dried with anhydrous Na2SO4. Filtered and concentrated under reduced pressure. Purification by column chromatography gave the desired Compound 9 (Compound D). Characterization of these compounds matched with prior literature. H NMR (400 MHz, CDCl3) δ 6.81 (br, s, 3H), 5.78 (s, 1H), 5.42 – 5.26 (m, 2H), 4.37 (d, 1 J = 5.6 Hz, 2H), 3.86 (s, 6H), 2.26 – 2.16 (m, 3H), 1.99 (dd, J = 13.6, 6.9 Hz, 2H), 1.66 (dt, J = 15.4, 7.6 Hz, 2H), 1.39 (dt, J = 15.1, 7.6 Hz, 2H), 0.95 (d, J = 6.8 Hz, 6H). 12 C NMR (101 MHz, CDCl3) δ 172.76 (s), 149.22 (s), 148.51 (s), 138.09 (s), 131.13 (s), 13 126.48 (s), 120.08 (s), 111.28 (s), 111.26(s), 55.95 (s), 55.89(s), 43.40 (s), 36.69 (s), 32.21 (s), 30.95 (s), 29.29 (s), 25.27 (s), 22.63 (s). MS Calculated for C19H30NO3 [M+H] + 320.2, found 320.5. 13