Liver Lab - Spring Woods Pre

Liver Lab
Objective
In this experiment, you will extract the enzyme catalase from liver, use it to break down hydrogen peroxide, and test the activity of catalase under different conditions.
Introduction
A chemical reaction is when chemical come together and their molecules interact to form new chemicals. Sometimes chemical reactions happen by themselves. These reactions are usually very fast and spontaneous, and give off energy.
Other chemical reactions need energy to happen, and without energy proceed very slowly or not at all.
These types of chemical reactions can be helped to occur more quickly by using enzymes. Enzymes are made out of protein and they speed up the rate of a chemical reaction by acting as a catalyst. A catalyst provides the necessary environment for the chemical reaction to occur, which speeds up the reaction. Certain catalysts work for certain kinds of reactions, in other words each enzyme has a particular type of reaction that it can activate.
Enzymes are proteins, which are molecules that are very large and dynamic. They can be very fussy, and sometimes need to be in certain environments or conditions to work. Some enzymes can be damaged under certain conditions such as heat and change in pH. A damaged enzyme will no longer work to catalyze a chemical reaction.
One source of enzymes is the liver, which needs to break down many substances in the body. Catalase is
one enzyme from liver that breaks down harmful hydrogen peroxide into oxygen gas and water:
2H
2
O
2
------> 2H
2
0 + O
2
When this chemical reaction occurs, you can see the oxygen gas bubbles escaping and causing the reaction to foam.
In this experiment, you will use liver as a source of catalase and investigate how the activity of the enzyme can change under certain conditions. What does catalase do? Under which conditions does it work best? Why do we need catalase in our liver?
Part 1: Pre Lab
1. What pH would catalase work best at if it is found in the liver?
2. What temperature would catalase work best at if it is found in the liver?
Part 2: Lab
Problem:________________________________________________________________________________
Hypothesis:_______________________________________________________________________________
_________________________________________________________________________________________
Materials:
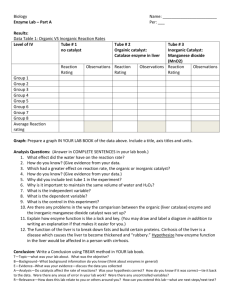
4 test tubes Hydrogen Peroxide 4 pieces of liver Dropper Ruler Marker
Procedure:
1. Put one dropper full of peroxide into all 4 test tubes.
2. In one test tube add a piece of fresh liver
3. Make a mark at highest point on the test tube that the bubbles reached.
4. Measure in cm from the bottom of your test tube to you mark and record your data on the chart.
5. Repeat for cooked liver and bleached liver
Test Tube
No Liver
Raw Liver
Cooked Liver
Bleached Liver
Height of Bubbles in CM
Conclusion:
1. What is the name of the enzyme in this experiment?
2. What is the substrate?
3. What are the products?
4. What gas was being released? (What were the bubbles?)
5. Draw a diagram to illustrate the chemical reaction that took place in your lab; it should include the enzyme, substrate and products. (Make sure you label!!!!)
6. Explain what caused the differences in bubble production between the cooked liver, raw liver and bleached liver. Make sure to address the changes in pH and temperature.
7. Write a conclusion for this lab. Make sure to include your hypothesis, whether or not you were correct and
DATA that supports your findings. WRITE IN COMPLETE SENTENCES.