HL ONLY - uaschemistry
advertisement
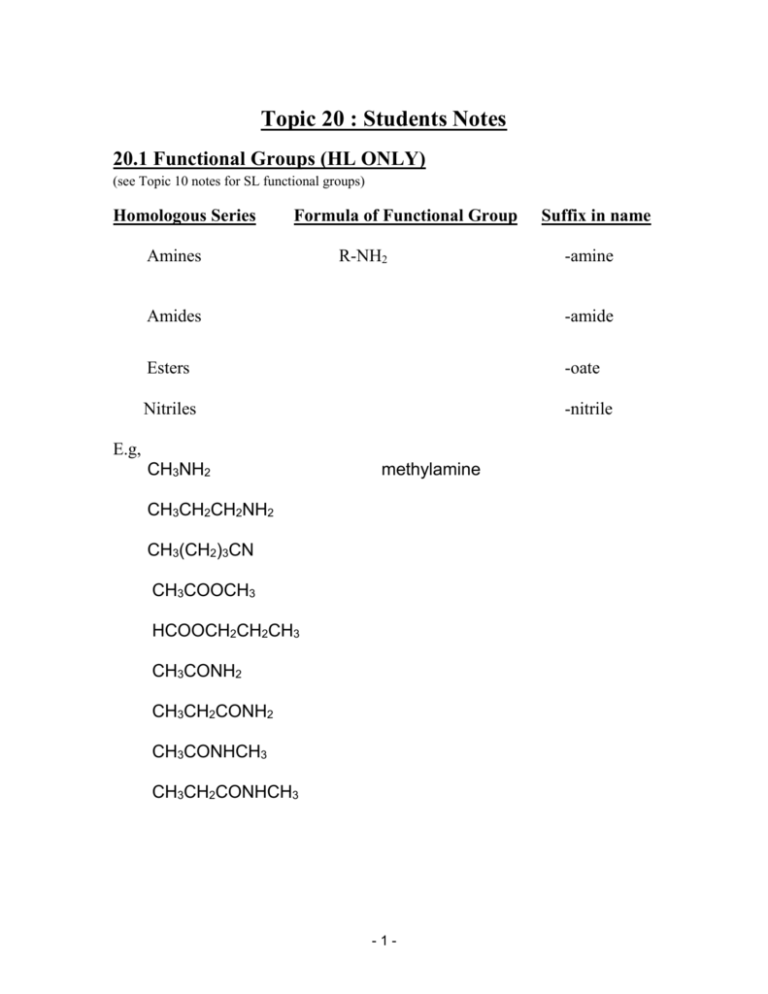
Topic 20 : Students Notes 20.1 Functional Groups (HL ONLY) (see Topic 10 notes for SL functional groups) Homologous Series Formula of Functional Group Amines R-NH2 Suffix in name -amine Amides -amide Esters -oate Nitriles -nitrile E.g, CH3NH2 methylamine CH3CH2CH2NH2 CH3(CH2)3CN CH3COOCH3 HCOOCH2CH2CH3 CH3CONH2 CH3CH2CONH2 CH3CONHCH3 CH3CH2CONHCH3 -1- 20.2 Nucleophilic Substitution Reactions of Halogenoalkanes (HL ONLY) Nucleophiles are chemical species that are able to provide an outer electron pair to an electron deficient species. In halogenoalkanes the electron deficient species is the carbon atom in the polar C-X bond. Substitution occurs when the C-X bonds breaks (heterolytically) and a new bond forms with the nucleophile. Factors Affecting the Rate of Nucleophilic Substitution 1. Identity of the halogen. Bond breaking is an endothermic process. The stronger the C-X bond, the more energy required to break this bond, the less energetically favourable the reaction. C-I bonds are the weakest and break the easiest, then CBr bonds and C-Cl bonds are the strongest Iodoalkanes react the fastest then bromoalkanes and then chloroalkanes. 2. Type of Nucleophile. Bond forming is an exothermic process. The stronger the bond formed between the nucleophile and the halogenoalkane, the more energetically favourable the reaction. -2- Hydroxide ions are therefore better nucleophiles than water because no bond breaking is required in the process. Hydroxide (OH-) ions react faster than water (H2O). 3. The type of halogenoalkane. The reaction involving intermediate carbocations (SN 1) reacts faster than the formation of the activated complex (SN2). This is because the formation of an activated complex requires a larger activation energy (collision energy). Tertiary halogenoalkanes react fastest then secondary and primary react the slowest. 20.2 Other Nucleophilic Reactions of Halogenoalkanes (HL ONLY) Nucleophiles have non bonded electron pairs that they are able to donate. The easier they can do this, the more effective the nucleophile. NH3, CN-, OH- are ‘good’ nucleophiles. Cl- and H2O are not as effective nucleophiles. The nucleophile attacks the slightly positive carbon atom bonded to the halogen. This causes the polar C-X bond to break so that the halogen is substituted by the nucleophile. E.g. 1. Reaction with NH3 to give amines. -3- E.g. 2. Reaction with KCN to give nitriles. Note: The nitriles produced in these reactions can be reduced using hydrogen (in the presence of a Ni catalyst) to produce an amine. E.g. Substitution of a halogenoalkene with CN- and then reduction of the product with H2 (Ni) is an especially useful reaction pathway, as it increases the length of the carbon chain by one. These examples of nucleophilic substitution can also be described in terms of SN1 and SN2 mechanisms. What type of mechanism would be used in the reaction between iodoethane CH3CH2I and potassium cyanide KCN? Give the rate expression for this reaction. Draw the mechanism expected in the nucleophilic substitution reaction between bromoethane CH3CH2Br and ammonia NH3. Label the rate determining step. -4- 20.3 Elimination Reactions of Halogenoalkanes (HL ONLY) Refluxing bromoalkanes with a conc solution of hydroxide (OH) ions (dissolved in ethanol). Results in the elimination of water to give an alkene. E.g. 2-bromopropane and NaOH(in ethanol). The OH- ion acts as a base. Removing a hydrogen ion to give water and resulting in the release of the bromide ion from the molecule. Mechanism: Show the mechanism for the reaction of 1-bromoethane reacting with conc KOH dissolved in ethanol. -5- 20.4 Condensation Reaction (HL ONLY) a) Carboxylic Acids and Alcohols Alcohols react with carboxylic acids to produce sweet smelling compounds called esters. During the reaction, the alcohol and acid condense together and eliminate water. This is therefore referred to as a condensation reaction. E.g. Ethanoic acid and methanol. This reaction is an equilibrium (reversible). An acid (H+) catalyst is used usually in the form of conc H2SO4. This H+ has a dual purpose. It acts as a catalyst to speed up the rate of reaction and it maximises the yield of ester by shifting the equilibrium position to the right. Esters are commonly used as flavouring in food and in perfumes. E.g.2 Give the equation for the reaction between methanoic acid and ethanol. -6- Condensation Polymers of Carboxylic Acid/Alcohols. Carboxylic acids will also form polymer structures with alcohols. Both the acid and the alcohol must contain 2 functional groups. A di-oic acid and a di-ol) Unlike the addition polymerisation reactions of the alkenes, condensation polymerisation involves the elimination of a water (or sometimes an HCl) molecule. The polymer formed is called a polyester. The most common polyester is called Terylene or Dacron. E.g. -7- b) Carboxylic acids and Ammonia/Amines Carboxylic acids react with ammonia and amines to give an amide molecule and water. They are therefore also condensation reactions. E.g. propanoic acid and ammonia E.g. ethanoic acid and methylamine Condensation Polymerisation of Carboxylic acid/Amines Carboxylic acids can form polymer structures by reaction with amines. In order for a polymer to form, the carboxylic acid and the amine must both contain 2 functional groups. (A d-ioic acid with a di-amine) Water is also eliminated and so this is also a condensation reaction. The polymer formed is called a polyamide. The most common polyamide is nylon. E.g. -8- 20.6 Steroisomerism (HL ONLY) Isomers have the same molecular and structural formula but differ in the arrangement of the atoms in space (spatial arrangement). There are 2 types of stereoisomerism: Geometric and optical. 1. Geometric Isomers a) Geometric isomers in alkene molecules. In alkenes, there is restricted rotation around the double bond C=C. This bond consists of a sigma and pi bond. The sigma bond (formed by interaction of hybrid bonding orbitals) allows the atoms to rotate fully. The pi bond (formed by the parallel interaction between atomic p-orbitals above and below the plane of the molecule) cannot allow rotation of the atoms. This means that 2 isomers can exist. One isomer has both groups attached on the same side of the double bond. This is called a cis isomer. The second isomer has groups attached on opposite sides of the molecule. This is called a trans isomer. E.g. But-2-ene E.g. 1, 2, dichloroethene. -9- Physical properties, such as boiling points can be greatly affected by geometric isomerism. E.g. cis-1,2-dichloroethene boils at a much higher temperature (600) than its trans isomer (480). This happens because the strength of the intermolecular forces between the molecules is affected by the orientation of the polar (dipole) bonds. Chemical properties can also be affected, but this is less common. If two functional groups are on the same side of the molecule in a cis-isomer, they can sometimes interact causing a chemical reaction. This happens in cis-but-2-ene-1,4-dioic acid, which decomposes at 1300 whereas the trans isomer does not. In the cis-isomer, the acid functional groups interact with each other forming a hydrogen bond. When heated, the formation of this hydrogen bond causes the atoms to be close enough together to react. In the trans-isomer this cannot happen as the groups are on opposite sides and its forms normal hydrogen bonds with other molecules resulting in it having a very high melting point without decomposing. (2860) b) Geometric Isomers in Cyclo-Alkanes An alkane that forms a ring structure is called a cyclo-alkane. A ring structure also has a restricted rotation of its bonds. This also leads to the formation of geometric isomers. - 10 - If both groups are attached above or below the ring structure this is a cis-isomer. If one group is above the ring structure and one group below, then this will be a trans-isomer. E.g. cis- dichloro-cyclo-propane trans-dichloro-cyclo-propane E.g. cis-dichloro-cyclo-butane trans-dichloro-cyclo-buatne 2. Optical Isomerism (HL ONLY) For a molecule to have optical isomers, it must have a carbon atom that is bonded to 4 different atoms or molecules. This is called an asymmetric (or chiral) carbon atom. E.g. Butan-2-ol This type of molecule can exist as a mirror image of itself. - 11 - A molecule (such as butan-2-ol) that is able to exist in these two different forms is said to be chiral. Label the asymmetric carbon atom in 2-bromobutane (CH3CHBrCH2CH3) and then draw the optical isomers. Properties of Optical Isomers Each optical isomer is called an enantiomer. Each enantiomer has exactly the same physical properties except for their effect on plane polarised light. One enantiomer will rotate the light clockwise and is called the (D) or (+) enantiomer. The other enantiomer will rotate the light anticlockwise and is called the (L) or the (-) enantiomer. Most substances have an equal mix of the 2 isomers and this is called a racemic mixture. A racemic mixture will have - 12 - no effect on plane polarised light because the effects will cancel out. Each enantiomer has exactly the same chemical properties except for how they react with other optical isomers. Many of the 2-amino acids in the body are optically active in this way and will react in different ways to other optically active substances. (Stereo specific) Proteins contain only L-amino acids, whereas muscles can produce only D-Lactic acid. Drugs and medicines have to be sensitive to optical activity. E.g. the drug thalidomide is a racemic mixture of 2 enantiomers, one of which reduces the effects of morning sickness, the other catalyses reactions that leads to deformation of unborn foetus’. TOK: How does the existence of optical isomers give evidence of the tetrahedral bonding in carbon molecules? - 13 - 20.5 Reaction Pathways (HL ONLY) Deduce the two stage reaction pathways for the following organic conversions, given the starting materials and products: Include the reagents and conditions required. Starting Material Product 1-chloroethane propylamine Propene propanoic acid Methanal Ethyl Methanoate 1-bromopropane 1,2-dichloropropane 1-Iodobutane Butyl methanamide Ethanol Methyl ethanamide Adapted from © Chemistry@ Shatin College, Hong Kong. - 14 -








