Study guide - Cloudfront.net
advertisement
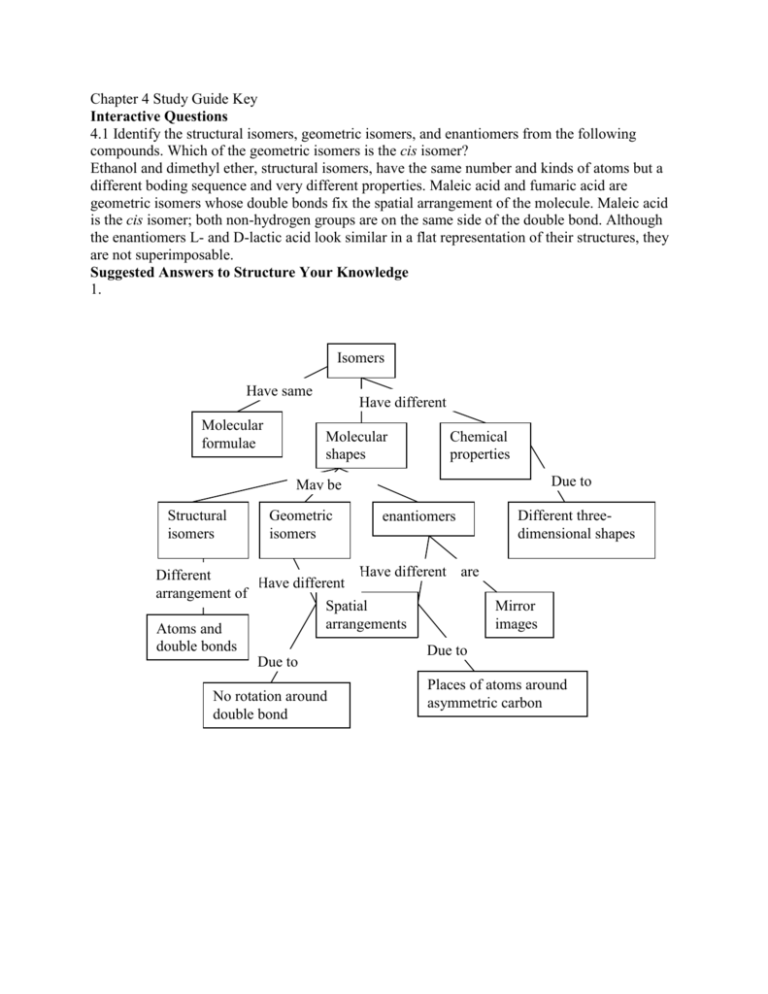
Chapter 4 Study Guide Key Interactive Questions 4.1 Identify the structural isomers, geometric isomers, and enantiomers from the following compounds. Which of the geometric isomers is the cis isomer? Ethanol and dimethyl ether, structural isomers, have the same number and kinds of atoms but a different boding sequence and very different properties. Maleic acid and fumaric acid are geometric isomers whose double bonds fix the spatial arrangement of the molecule. Maleic acid is the cis isomer; both non-hydrogen groups are on the same side of the double bond. Although the enantiomers L- and D-lactic acid look similar in a flat representation of their structures, they are not superimposable. Suggested Answers to Structure Your Knowledge 1. Isomers Have same Molecular formulae Have different Molecular shapes Chemical properties Due to May be Structural isomers Geometric isomers enantiomers Have different are Different Have different arrangement of Spatial arrangements Atoms and double bonds Due to Due to No rotation around double bond Different threedimensional shapes Mirror images Places of atoms around asymmetric carbon 2. Functional Group Hydroxyl Carbonyl Carboxyl Amino Sulfhydryl Phosphate Molecular Formula -OH =C=O -COOH -NH2 -SH -OPO32- Names and Characteristics of Organic Compounds Containing Functional Group Alcohols; polar group Aldehyde or ketone; polar group Carboxylic acid; release H+ Amines; basic, accept H+ Thiols; cross-links stabilize protein structure Organic phosphates; used in energy transfers, acidic Answers to Test Your Knowledge Multiple Choice 1. c; its four electrons in the valence shell that can form four covalent bonds. (p60) 2. b; the C-H bond is nonpolar. (p62) 3. e; It can be a part of geometric isomers. (p62) 4. a; mechanism (p59) 5. d; -NH2 (p64) 6. a; -COOH (p64) 7. d; They are a result of restricted movement around a carbon double bond. (p62) 8. d; hydrocarbons (p61) 9. c; 3 (p62) Matching (refer to pages 64-65) 1. a, c 2. b, f 3. a, d, e 4. e 5. e 6. a, c, d, e 7. bf 8. e 9. ac 10. a, d 11. e 12. c