Graphing Periodic Trends
advertisement

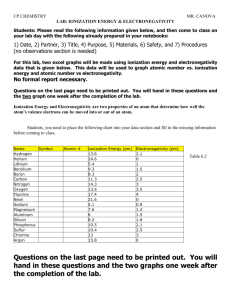
Lab Activity: Periodicity of Properties of the Elements on Microsoft Excel Background The periodic table is an arrangement of elements based on atomic number. The modern Periodic Table was developed in 1913 by Henry Moseley. He used X-rays to identify the atomic number of each element. When elements are arranged in order of increasing atomic number, their physical and chemical properties show a periodic pattern. There are many measurements that help us predict the chemical behavior of an element. These measurements are all related to either the number of valence electrons of the attraction between the valence electrons and the nucleus of an atom. These measurements include Ionization Energy, Electronegativity, and Atomic Radius. At the end of this lab you will each be handing in print outs of your data and graphs along with your written conclusion. Purpose To plot several properties of elements and observe the regularities in these properties Materials Chemistry Reference Table, Microsoft Excel Instructions 1) Open Microsoft Excel 2) Enter headings in each of the following cells A1: Element Symbol B1: Atomic Number C1: Ionization Energy D1: Electronegativity E1: Atomic Radius 3) Using your reference tables, enter the appropriate values for each column. You will record data for element 1-36 (H-Kr) For column D, Electronegativity: Enter “0” for noble gases He, Ne, Ar, Kr 4) You will analyze the data entered using three separate graphs! Graph 1: Atomic Number X series: B2:B37 vs. Ionization Energy Y series: C2:C37 Graph 2: Atomic Number X series: B2:B37 vs. Electronegativity Y series: D2:D37 Graph 3: Atomic Number X series: B2:B37 vs. Atomic Radius Y series: E2:E37 To construct each graph: a. Click on the letter for Atomic Number (B), (the whole column should be highlighted) then hold down the Control key and click on the letter C of the column for Ionization Energy. Both columns should be highlighted. b. Go to “Insert” and select “Chart”. Click on Chart Type “XY Scatter” and select a subtype that has the points connected by lines. c. Click on “Next>” d. Click on “Next>” again e. Under “Chart Title” click and type “(Your initials) Atomic Number vs. Ionization Energy” Make sure your partner’s and your initials are on the title. f. Click on “Category X axis” and type “Atomic Number” g. Click on “Category Y axis” and type “ Ionization Energy (kJ/mol)” h. Click on “Next>” i. Save as “New Sheet Chart 1” (Chart # will change with graphs) j. Click on “Finish” k. The chart is saved on Chart 1, on the bottom of the sheet click on Sheet 1. This should lead you back to the original data tables. l. Repeat for the two other graphs m. Print all of the sheets, including the data table. n. If you have not printed out all of your graphs by the end of the period, save your Excel file in the U: drive in the Falk folder. Conclusion Questions (To be handed in with the data): 1. On graph 1. Circle and identify the peaks and valleys on the graphs. Which groups tend to have high ionization energy? Which groups tend to have low ionization energy? 2. On graph 2. Circle and identify the peaks and valleys on the graphs. Which groups tend to have high electronegativity? Which groups tend to have low electronegativity? 3. On graph 3. Circle and identify the peaks and valleys on the graphs. Which groups tend to have the largest radius? Which groups tend to have the smallest radius? 4. What is the explanation of the trends observed across a period? (Discuss the relationship between nuclear charge, principal energy levels, and attraction between the nucleus and valence electrons) Conclusion (To be handed in): From this lab I learned…