What defines an element
advertisement
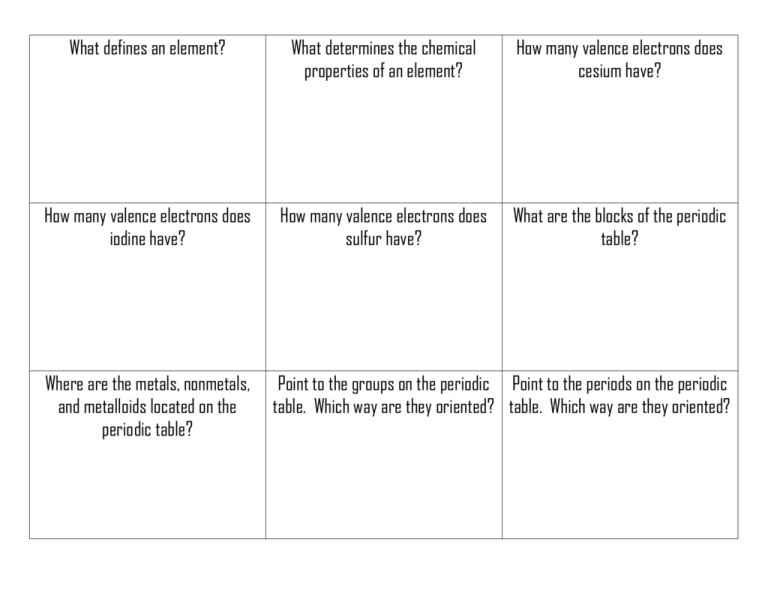
What defines an element? What determines the chemical properties of an element? How many valence electrons does cesium have? How many valence electrons does iodine have? How many valence electrons does sulfur have? What are the blocks of the periodic table? Where are the metals, nonmetals, and metalloids located on the periodic table? Point to the groups on the periodic Point to the periods on the periodic table. Which way are they oriented? table. Which way are they oriented? What does the group number tell you about an atom? What does the period number tell you about an atom? By what number is the periodic table organized? Where are the solids & gases located on the Periodic Table Which is a semi-metal: Cu, Mg, S, or Ge? . What are the two liquids on the periodic table? . How many protons does strontium (Sr) have? Which is a nonmetal: Cl, Rb, Al, As? What are the properties of metals? What are the properties of nonmetals? What are the properties of semimetals? What is the radius of atom? The radius for a negative ion increases or decreases? Why? The radius for a positive ion increases or decreases? Why? What is electronegativity? What is ionization energy? When sulfur forms an ion, does its radius get larger or smaller? Why? What is periodicity? Which has a greater electronegativity: K or Br? Which has a greater ionization energy: K or Br? Which has a greater atomic radius: K or Br? Which has a greater electronegativity: Be or Ra? Which has a greater ionization energy: Be or Ra? Which has a greater atomic radius: Be or Ra? Why does atomic radius increase top Why does electronegativity decrease to bottom? top to bottom? Why does atomic radius decrease left to right? Will oxygen and selenium behave the same? Why or why not? Why does ionization energy decrease top to bottom? Why does electronegativity increase Why does ionization energy increase left to right? left to right? Will boron and fluorine behave the same? Why or why not? Will lithium and rubidium behave the same? Why or why not? When you see an ACT passage with multiple opinions presented, what kind of passage is this? When you see an ACT passage with several versions of an experiment presented, what kind of passage is this? In what order do we complete ACT passages? BOOM! BOOM! BOOM! BOOM! BOOM! BOOM!







