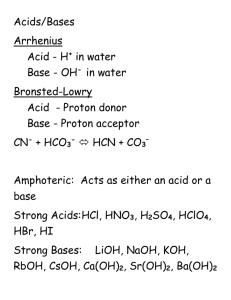
Acids and Bases Review
advertisement

PQ – Acids and Bases in All Different Places Hour______ Date______ Name____________________________ 1. Definitions Ionic Compound Molecular Compound Acid Base Salt Electrolyte Neutralization Indicator Buffer Titration o Standard Solution o Unknown Solution 2. What are the 5 properties of acids and bases? Acids 1 2 3 4 5 Bases PQ – Acids and Bases in All Different Places Hour______ Date______ Name____________________________ 3. Draw the Lewis Structures for the following substances. For each: Give the name of the substance Label it as an acid, base, salt, or neither a. H2S b. NaOH c. NaCl d. SCl2 4. Rank the following acids from weakest to strongest: H2SO4, HF, H3PO4, HCl 5. Name the following compounds, and label as an acid (A), base (B), salt (S), or neither (N): ____________________________ a. CaCl2 _________________________ d. LiOH ____________________________ b. HNO3 _________________________ e. Li2O ____________________________ c. Mg(OH)2 _________________________ f. CO2 6. Give the formulas for the following compounds, and label as an acid (A), base (B), salt (S), or neither (N): ___________________ a. barium bromide ________________ d. aluminum hydroxide ___________________ b. hydrobromic acid ________________ e. dinitrogen monoxide ___________________ c. sodium hydroxide _________________ f. sulfuric acid 7. Give the products for the following neutralization reactions. Label the acid, base, salt, and water. a. _____ HBr(aq) + _____ NaOH(aq) b. _____ HCl(aq) + _____ Mg(OH)2(aq) PQ – Acids and Bases in All Different Places Hour______ Date______ Name____________________________ 8. Given the information below, label the solutions as acidic, basic, or neutral. What color would each show with pH paper? pH acidic, basic, neutral a) 3.8 b) 5.8 c) 7.8 color pH acidic, basic, neutral color d) 7.0 e) 13.4 f) 7.1 9. Using the information in #8, answer the following questions: a. Which is most acidic? _______________ least acidic? _____________ b. Which is most basic? _______________ least basic? _____________ c. (a) is ________ times less acidic than (b) and ________ times less acidic than (c). 10. Look at the data in the table below and answer the questions: Substance initial pH pH after 1 drop HCl pH after 2 drops NaOH A 10.7 10.2 11.2 B 4.4 4.3 4.5 C 7.0 1.9 12.4 PQ – Acids and Bases in All Different Places Hour______ Date______ Name____________________________ a. Using your indicator table in the notes, give the color that each of the substances will show before adding any acid or base: Substance initial pH A 10.7 B 4.4 C 7.0 acid, base, or neutral? color in methyl red color in phenolphthalein b. Look at the 3rd column of the top table. What is the Lewis Structure for HCl? Is it an acid or a base? When you add HCl, what happens to the pH? c. Look at the 4th column of the top table. What is the Lewis Structure for NaOH? Is it an acid or a base? When you add NaOH, what happens to the pH? d. Which solution is the best buffer: A, B, or C? Explain your answer. 11. If you titrate some vinegar (weak acid) of unknown concentration with a standard 0.800 M solution of NaOH, what is the concentration of vinegar if the titration required: a. 5.0 mL of vinegar and 7.6 mL of NaOH d. exactly 5 drops of vinegar and 31 drops of NaOH